Online Database of Chemicals from Around the World
| Jinxiang Chemical (Danyang) Products Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8681-9862 +86 13913839793 | |||
 |
sales@jinchemical.com nancy-zong@hotmail.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Kingfirst Chemical (Nanjing) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8452-2992 8477-3604 | |||
 |
marketing@kingfirstchem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Dongtai Xinyuan Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (515) 8548-3100 (21) 5289-2866 | |||
 |
sales@xin-yuanchem.com xuxiaogang@9.cn | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2007 | ||||
| Xuzhou Ruisai Technology Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (526) 8776-8018 | |||
 |
sales@risachem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Palchem | France | Inquire | ||
|---|---|---|---|---|
 |
+33 (3) 2145-2678 | |||
 |
palchem@wanadoo.fr | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Classification | Organic raw materials >> Hydrocarbon compounds and their derivatives >> Hydrocarbon halide |
|---|---|
| Name | Benzyl bromide |
| Synonyms | alpha-Bromophenylmethane; alpha-Bromotoluene; Bromomethylbenzene; a-Bromotoluene |
| Molecular Structure | 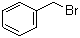 |
| Molecular Formula | C7H7Br |
| Molecular Weight | 171.04 |
| CAS Registry Number | 100-39-0 |
| EC Number | 202-847-3 |
| SMILES | C1=CC=C(C=C1)CBr |
| Density | 1.4±0.1 g/cm3 Calc.*, 1.438 g/mL (Expl.) |
|---|---|
| Melting point | -3 - -1 ºC (Expl.) |
| Boiling point | 198.5 ºC 760 mmHg (Calc.)*, 198 - 199 ºC (Expl.) |
| Flash point | 86.7 ºC (Calc.)*, 86 ºC (Expl.) |
| Index of refraction | 1.568 (Calc.)*, 1.575 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| Transport Information | UN 1737 | ||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||
|
Benzyl bromide is an aromatic alkyl halide with the molecular formula C7H7Br, consisting of a bromomethyl group directly attached to a benzene ring. It is a colorless to pale yellow liquid with a strong, irritating odor and is known for its high reactivity toward nucleophiles. The compound occupies an important place in organic chemistry as one of the simplest and most widely used benzylating agents, enabling the introduction of benzyl groups into a broad range of molecules. The discovery and early study of benzyl bromide are closely connected to the development of aromatic chemistry in the late nineteenth and early twentieth centuries. As methods for the halogenation of alkylbenzenes became established, chemists identified benzyl bromide as a readily accessible compound formed by substitution at the benzylic position. Its structure and reactivity were soon recognized as distinct from those of aryl halides, because the benzylic carbon readily undergoes substitution reactions. This behavior was explained by the stabilization of the benzyl cation and transition states through resonance with the aromatic ring, a concept that later became fundamental in physical organic chemistry. The preparation of benzyl bromide has traditionally relied on the bromination of toluene or benzyl alcohol derivatives. Early laboratory methods involved direct reaction of toluene with bromine under conditions favoring substitution at the side chain rather than the aromatic ring. Later refinements introduced more controlled approaches, such as radical bromination using reagents that selectively abstract benzylic hydrogen atoms. These developments made benzyl bromide a standard reagent that could be prepared reproducibly on both laboratory and industrial scales. The primary application of benzyl bromide is as an alkylating agent in organic synthesis. It reacts readily with nucleophiles such as alcohols, phenols, thiols, amines, and carboxylates to form benzyl ethers, thioethers, amines, and esters. This reactivity has made it indispensable in protecting group chemistry, where benzyl groups are commonly used to mask functional groups during multistep syntheses. The benzyl protecting group can later be removed under well-defined conditions, allowing selective deprotection without disturbing other parts of a molecule. In medicinal and pharmaceutical chemistry, benzyl bromide has been widely used to introduce benzyl substituents into drug candidates and intermediates. Benzyl groups can modify lipophilicity, metabolic stability, and binding properties of biologically active molecules. As a result, benzyl bromide appears frequently in synthetic routes toward active pharmaceutical ingredients, agrochemicals, and fine chemicals. Its straightforward reactivity allows efficient carbon–heteroatom bond formation, which is central to constructing complex molecular frameworks. Benzyl bromide has also played a role in the development of reaction mechanism theory. Its rapid participation in nucleophilic substitution reactions made it a model substrate for studying reaction kinetics and solvent effects. Investigations into its behavior contributed to the understanding of SN1 and SN2 mechanisms, particularly in systems where resonance stabilization influences reaction pathways. These studies helped establish principles that are now standard in organic chemistry education. Despite its usefulness, benzyl bromide is recognized as hazardous. It is a strong lachrymator and skin irritant, and it can cause severe respiratory and eye irritation upon exposure. Prolonged or repeated contact poses additional health risks. Consequently, its handling requires appropriate safety measures, including adequate ventilation and protective equipment. These properties have encouraged the development of alternative reagents and milder conditions, although benzyl bromide remains widely used due to its effectiveness. Overall, benzyl bromide is a historically significant and practically indispensable reagent in organic chemistry. From its early identification as a benzylic halide to its extensive application in synthesis, protection strategies, and mechanistic studies, it has contributed substantially to both theoretical understanding and practical methodology. Its continued use reflects a balance between its high reactivity and the careful controls required for safe handling. References Carey FA, Sundberg RJ (2007) Advanced Organic Chemistry, Part A: Structure and Mechanisms. 5th edn. Springer DOI: 10.1007/978-0-387-44899-2 |
| Market Analysis Reports |
| List of Reports Available for Benzyl bromide |