2-Chloroethanol, also known as ethylene chlorohydrin, is a simple chlorinated alcohol with the molecular formula C2H5ClO. Its discovery dates back to the early 19th century during research on alcohol derivatives. The compound was identified as a byproduct in various chemical reactions involving ethylene and chlorine, marking its importance in the chemical industry. With its reactive hydroxyl group and chlorine atom, 2-chloroethanol plays a pivotal role as a chemical intermediate, particularly in the synthesis of larger molecules.
One of the primary applications of 2-chloroethanol is in the production of ethylene oxide, a significant industrial chemical used to manufacture a variety of products including antifreeze, detergents, and solvents. 2-Chloroethanol reacts with a base, typically sodium hydroxide, to yield ethylene oxide through an intramolecular cyclization process. This reaction has been widely used in industrial processes for the large-scale production of ethylene oxide, which in turn serves as a precursor for producing ethylene glycol.
Another key application of 2-chloroethanol is its use in the preparation of pharmaceuticals and agrochemicals. It serves as an intermediate in the synthesis of compounds with biologically active properties, including pesticides, herbicides, and certain pharmaceutical agents. Its ability to introduce a reactive chloroethyl group into organic molecules has been exploited in the production of specialty chemicals that require specific structural features.
In the realm of organic synthesis, 2-chloroethanol can be used as an alkylating agent. Its reactivity allows it to introduce ethoxy groups into various chemical structures, thereby enabling the modification of molecular frameworks in both research and industrial chemistry. Additionally, it has found applications in the production of plasticizers and surfactants, where it acts as a building block for more complex chemicals.
While 2-chloroethanol has proven valuable in industrial and chemical applications, it is important to note that it is a toxic and potentially hazardous substance. Its harmful effects on human health are primarily due to its irritant and neurotoxic properties. Inhalation or dermal exposure can lead to symptoms such as dizziness, headaches, and respiratory distress. Consequently, strict handling and safety protocols are in place when working with 2-chloroethanol, particularly in industrial settings.
Despite these hazards, 2-chloroethanol remains a crucial component in various chemical industries due to its versatility as a chemical intermediate. Its ability to contribute to the synthesis of diverse compounds, from pharmaceuticals to industrial chemicals, underscores its significance in modern chemistry.
|










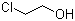
 GHS06 Danger Details
GHS06 Danger Details