Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Chemical reagent >> Organic reagent >> Ester >> Butyl ester compound |
|---|---|
| Name | tert-Butyl chloroacetate |
| Molecular Structure | 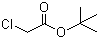 |
| Molecular Formula | C6H11ClO2 |
| Molecular Weight | 150.60 |
| CAS Registry Number | 107-59-5 |
| EC Number | 203-506-1 |
| SMILES | CC(C)(C)OC(=O)CCl |
| Density | 1.1±0.1 g/cm3 Calc.*, 1.053 g/mL (Expl.) |
|---|---|
| Boiling point | 157.5 ºC 760 mmHg (Calc.)*, 177.4 - 178.8 ºC (Expl.) |
| Flash point | 46.7 ºC (Calc.)*, 47 ºC (Expl.) |
| Solubility | water: <0.1 g/L (20 ºC) (Expl.) |
| Index of refraction | 1.425 (Calc.)*, 1.423 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |




 GHS02;GHS05;GHS06;GHS07;GHS09 DangerGHS02 Details
GHS02;GHS05;GHS06;GHS07;GHS09 DangerGHS02 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H226-H302+H312-H302-H312-H314-H315-H317-H319-H331-H400-H410 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P260-P261-P264-P264+P265-P270-P271-P272-P273-P280-P301+P317-P301+P330+P331-P302+P352-P302+P361+P354-P303+P361+P353-P304+P340-P305+P351+P338-P305+P354+P338-P316-P317-P321-P330-P332+P317-P333+P317-P337+P317-P362+P364-P363-P370+P378-P391-P403+P233-P403+P235-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3272 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
tert-Butyl chloroacetate is an ester of chloroacetic acid with the molecular formula C6H11ClO2. Structurally, it consists of a chloroacetyl group linked to a tert-butyl ester moiety, combining a reactive halogenated methylene group with a bulky, acid-labile protecting group. Under ambient conditions, tert-butyl chloroacetate is typically a colorless liquid with a characteristic ester odor. It is soluble in common organic solvents and exhibits limited solubility in water. The compound is widely used as a synthetic intermediate in organic chemistry. The presence of the chloro substituent on the methylene carbon activates the molecule toward nucleophilic substitution reactions, allowing displacement of the chloride by a variety of nucleophiles such as amines, thiols, or alkoxides. This reactivity makes tert-butyl chloroacetate a convenient reagent for introducing protected acetic acid units into more complex molecular frameworks. A key feature of tert-butyl chloroacetate is the tert-butyl ester group, which functions as a protecting group for carboxylic acids. The tert-butyl ester is stable under neutral and basic conditions but can be readily removed under mildly acidic conditions, typically generating isobutene and carbon dioxide as byproducts. This property is particularly valuable in multistep synthesis, where selective protection and deprotection of carboxyl groups is required. In practice, tert-butyl chloroacetate is commonly employed in the synthesis of amino acid derivatives, peptides, and pharmaceutical intermediates. Alkylation of nitrogen- or sulfur-containing nucleophiles with this reagent introduces a protected carboxymethyl group, which can later be converted into the free acid. This strategy allows chemists to control functional group reactivity and improve overall synthetic efficiency. The compound can be prepared by esterification of chloroacetic acid with tert-butanol or by reaction of tert-butanol with chloroacetyl chloride under controlled conditions. Its handling requires standard precautions typical for halogenated esters, as the chloroacetyl moiety can be irritating and reactive toward biological nucleophiles. Overall, tert-butyl chloroacetate is a versatile and widely used reagent in organic synthesis. Its combination of a reactive chloroacetyl group and an acid-labile tert-butyl ester makes it especially valuable for constructing protected carboxylic acid derivatives and for enabling controlled functional group transformations in complex synthetic sequences. References 2018. Multi-colored electrochromic devices based on mixed mono- and bi-substituted 4,4'-bipyridine derivatives containing an ester group. Journal of Applied Electrochemistry, 48(6). DOI: 10.1007/s10800-018-1190-6 2015. A One-Pot and Efficient Synthesis of Zoledronic Acid Starting from Tert-butyl Imidazol-1-yl Acetate. Pharmaceutical Chemistry Journal, 49(1). DOI: 10.1007/s11094-015-1205-0 |
| Market Analysis Reports |
| List of Reports Available for tert-Butyl chloroacetate |