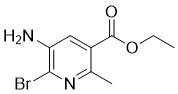
Ethyl 5-amino-6-bromo-2-methylnicotinate is a notable chemical compound with a unique structure and a variety of applications in research and industry. This molecule is a derivative of nicotinic acid, featuring a nicotinate core with an ethyl ester group, an amino group at position 5, a bromine atom at position 6, and a methyl group at position 2.
The discovery of Ethyl 5-amino-6-bromo-2-methylnicotinate stems from research into functionalized nicotinate derivatives, which are valued for their diverse biological activities and utility in medicinal chemistry. The combination of substituents—ethyl ester, amino group, bromine atom, and methyl group—adds distinct chemical properties to the molecule, making it a versatile candidate for various applications.
In medicinal chemistry, Ethyl 5-amino-6-bromo-2-methylnicotinate is of interest for its potential therapeutic properties. The nicotinate core is known for its biological activity, and the presence of the amino and bromine groups can significantly influence its pharmacological effects. The bromine atom may enhance the compound’s interaction with biological targets by affecting its electronic properties and steric effects. Researchers are exploring how these modifications impact the compound's activity against specific diseases, potentially leading to new drug candidates with enhanced efficacy and selectivity.
The compound also finds utility in organic synthesis. Its structure allows it to act as a valuable intermediate for synthesizing more complex molecules. The ethyl ester, amino group, and bromine atom contribute to its reactivity, enabling it to participate in various chemical transformations. This versatility is beneficial for developing novel compounds in pharmaceuticals and specialty chemicals.
Furthermore, Ethyl 5-amino-6-bromo-2-methylnicotinate serves as a useful reagent in the study of structure-activity relationships (SAR) in drug design. By examining how different substituents influence biological activity, researchers can gain insights into optimizing the design of therapeutic agents. The bromine atom, in particular, can provide valuable information on how halogenation affects drug activity and binding interactions.
In summary, Ethyl 5-amino-6-bromo-2-methylnicotinate is a compound with significant potential due to its unique structure and reactivity. Its applications in medicinal chemistry, organic synthesis, and SAR studies underscore its importance in advancing research and development in various scientific fields.
|


 GHS07 Warning Details
GHS07 Warning Details