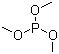
Trimethyl phosphite is an organophosphorus compound with the molecular formula C3H9PO3. Structurally, it consists of a phosphorus atom bonded to three methoxy groups (–OCH3), forming a trialkyl phosphite ester. It is a colorless, flammable liquid with a mild, characteristic odor, and it is soluble in most organic solvents while having limited solubility in water.
Trimethyl phosphite is primarily used as a reagent in organic synthesis. Its phosphorus center is nucleophilic, allowing it to participate in reactions with alkyl halides, acyl halides, and other electrophiles. One of its most notable applications is in the **Michaelis–Arbuzov reaction**, where trimethyl phosphite reacts with alkyl halides to produce dialkyl phosphonates. This transformation is widely employed in the preparation of organophosphorus compounds used in pharmaceuticals, agrochemicals, and as intermediates for further synthetic modifications.
The compound is also utilized in the synthesis of phosphonates, phosphinates, and other derivatives through nucleophilic substitution reactions. Its relatively low toxicity compared to other phosphorus reagents and its liquid state at room temperature make it convenient to handle in laboratory and industrial settings.
Trimethyl phosphite can be prepared by the reaction of phosphorus trichloride with methanol under controlled conditions. The reaction proceeds via substitution of the chlorine atoms with methoxy groups, yielding the trialkyl phosphite. Handling precautions are necessary, as it is flammable and can react with strong oxidizing agents.
Overall, trimethyl phosphite is a versatile organophosphorus reagent valued for its nucleophilic reactivity and ability to generate a range of phosphorus-containing products. Its use in the Michaelis–Arbuzov reaction and other substitution reactions makes it a cornerstone reagent in the synthesis of organophosphorus compounds for chemical and pharmaceutical research.
References
2025. Cobalt hydride-mediated photocatalytic semihydrogenation of acetylene impurities for continuous-flow production of polymer-grade ethylene. Nature Catalysis.
DOI: 10.1038/s41929-025-01380-z
2024. Photoinduced halogen anion-mediated arene C-H functionalization through arylsulfonium salts via electron donor-acceptor complexes. Science China Chemistry.
DOI: 10.1007/s11426-025-2682-y
2018. Rhenium and technetium complexes of thioamide derivatives of pyridylhydrazine that bind to amyloid-β plaques. Journal of Biological Inorganic Chemistry, 23(6).
DOI: 10.1007/s00775-018-1590-4
|



















 GHS02;GHS05;GHS07;GHS08 DangerGHS02 Details
GHS02;GHS05;GHS07;GHS08 DangerGHS02 Details