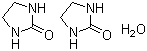
2-Imidazolidone hemihydrate is an heterocyclic urea derivative with the molecular formula C3H6N2O·0.5H2O. Structurally, it consists of a five-membered imidazolidone ring containing a carbonyl group at the 2-position and two nitrogen atoms at the 1- and 3-positions. The hemihydrate form indicates that one molecule of water is associated with two molecules of 2-imidazolidone in the crystal lattice, which can influence its crystallinity and solubility. The compound typically appears as a white crystalline solid, soluble in water and polar organic solvents such as methanol and ethanol.
2-Imidazolidone hemihydrate is primarily used as a building block in organic synthesis and pharmaceutical chemistry. The carbonyl and nitrogen functionalities provide reactive sites for nucleophilic addition, condensation, and ring-opening reactions, enabling the preparation of a variety of substituted imidazolidones, ureas, and heterocyclic derivatives. These derivatives are often used as intermediates in the synthesis of bioactive molecules, pharmaceuticals, and agrochemicals.
The compound also finds applications as a stabilizer and solvent additive in polymer chemistry. Its cyclic urea structure allows it to act as a hydrogen-bond donor and acceptor, which can influence polymer morphology, crystallinity, and solubility. It is sometimes used to enhance the performance of polyurethanes, epoxy resins, and other polymeric systems.
2-Imidazolidone hemihydrate can be synthesized by the cyclization of ethylenediamine with urea under controlled heating, with careful control of water content to obtain the hemihydrate form. Handling requires standard laboratory precautions, including avoiding ingestion, inhalation, and contact with skin or eyes, as the compound may cause irritation.
Overall, 2-imidazolidone hemihydrate is a versatile heterocyclic urea with reactive nitrogen and carbonyl functionalities. Its combination of solubility, reactivity, and hydrogen-bonding ability makes it a valuable intermediate in organic synthesis, pharmaceuticals, and polymer applications.
References
Xu K (2004) Nonaqueous liquid electrolytes for lithium-based rechargeable batteries. Chemical Reviews 104 10 4303–4418 DOI: 10.1021/cr030203g
|











 GHS07 Warning Details
GHS07 Warning Details