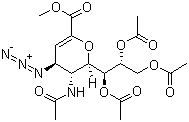
Methyl 5-acetamido-7,8,9-O-triacetyl-2,6-anhydro-4-azido-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonate is a complex chemical compound that has captured the attention of researchers in the fields of organic chemistry and biochemistry due to its unique structural features and potential applications. This compound is a derivative of a nonulosonic acid and incorporates azide functionality, which is significant in various chemical reactions, particularly in click chemistry.
The discovery of this compound stems from ongoing research into modified sugars and their derivatives, which have demonstrated promising biological activities. Its structural configuration includes multiple acetyl groups, which enhance its stability and reactivity. The synthesis of methyl 5-acetamido-7,8,9-O-triacetyl-2,6-anhydro-4-azido-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonate typically involves a multi-step process starting from simpler sugar precursors, followed by acetylation and azidation reactions.
One of the notable applications of this compound lies in its use as a glycosyl donor in carbohydrate chemistry. The azido group allows for easy incorporation into larger biomolecules through click chemistry, a powerful tool that enables the selective and efficient formation of chemical bonds. This capability is particularly valuable in the synthesis of glycoproteins, glycolipids, and other complex carbohydrates, which are critical in various biological processes and therapeutic applications.
Furthermore, the compound's potential extends to the development of targeted drug delivery systems. By modifying its structure to attach therapeutic agents or targeting moieties, researchers can create conjugates that enhance the specificity and efficacy of drug delivery to desired sites in the body. This is particularly relevant in cancer therapy, where targeted approaches can minimize off-target effects and improve treatment outcomes.
In addition to its synthetic applications, the compound has been investigated for its biological properties. Initial studies suggest that derivatives of this class may exhibit antimicrobial and anticancer activities. The presence of the azido group is known to enhance the reactivity of the compound in biological systems, potentially leading to the development of novel therapeutic agents that leverage these properties.
Safety and regulatory considerations are paramount when handling and studying compounds like methyl 5-acetamido-7,8,9-O-triacetyl-2,6-anhydro-4-azido-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonate. As with many azido compounds, caution is advised due to their potential explosiveness under certain conditions. Therefore, proper laboratory protocols and safety measures must be followed to mitigate risks during synthesis and application.
In conclusion, methyl 5-acetamido-7,8,9-O-triacetyl-2,6-anhydro-4-azido-3,4,5-trideoxy-D-glycero-D-galacto-non-2-enonate is a compound of significant interest in the fields of organic and medicinal chemistry. Its unique structure and functional groups offer versatile applications in carbohydrate synthesis, drug development, and potentially therapeutic agents. Ongoing research into its properties and applications may lead to valuable advancements in various scientific fields.
|

















 GHS07 Warning Details
GHS07 Warning Details