Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Win-Win Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (577) 6449-8589 +86 15325081899 | |||
 |
sales@win-winchemical.com winwinchemical@gmail.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical manufacturer since 2007 | ||||
| chemBlink standard supplier since 2011 | ||||
| Alfa Chemistry | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (201) 478-8534 | |||
 |
inquiry@alfa-chemistry.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2012 | ||||
| Nanjing Fred Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (25) 8469-6168 | |||
 |
Austin@fredchem.cn | |||
 |
Skype Chat | |||
 |
QQ chat | |||
 |
WeChat: NJFred01 | |||
 |
WhatsApp: +86 17302533743 | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Organic fluorine compound >> Fluorobenzonitrile series |
|---|---|
| Name | 4-Chloro-2,5-difluorobenzonitrile |
| Molecular Structure | 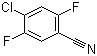 |
| Molecular Formula | C7H2ClF2N |
| Molecular Weight | 173.55 |
| CAS Registry Number | 135748-35-5 |
| SMILES | C1=C(C(=CC(=C1F)Cl)F)C#N |
| Density | 1.4±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 206.8±35.0 ºC 760 mmHg (Calc.)* |
| Flash point | 78.9±25.9 ºC (Calc.)* |
| Index of refraction | 1.513 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302 Details |
| Precautionary Statements | P280-P305+P351+P338 Details |
| SDS | Available |
|
4-Chloro-2,5-difluorobenzonitrile is an aromatic nitrile compound with the molecular formula C7H2ClF2N. It features a benzene ring substituted with a chlorine atom at the 4-position, fluorine atoms at the 2- and 5-positions, and a nitrile group at the 1-position. This arrangement results in a highly electron-deficient aromatic ring, which influences both its physical properties and chemical reactivity. The compound is generally found as a solid at room temperature and is characterized by moderate solubility in organic solvents such as dichloromethane and acetonitrile. This molecule belongs to the broader class of halogenated benzonitriles, which are widely used as building blocks in the synthesis of more complex organic molecules. The combination of halogens and a nitrile group makes 4-chloro-2,5-difluorobenzonitrile particularly suitable for nucleophilic aromatic substitution (S_NAr) reactions. These transformations are enabled by the electron-withdrawing nature of the nitrile and halogen substituents, which activate the aromatic ring toward nucleophilic attack at specific positions. The presence of multiple halogen atoms offers a platform for stepwise or selective substitution, allowing for diverse functionalization pathways. In synthetic chemistry, this compound serves as a versatile intermediate in the development of pharmaceuticals, agrochemicals, and specialty materials. For example, it is frequently employed in the preparation of substituted anilines or phenols via nucleophilic substitution, wherein a nucleophile such as an amine or alkoxide displaces one of the halogen atoms. The nitrile group can also be hydrolyzed to yield the corresponding carboxylic acid or converted into amides, amines, or other nitrogen-containing functionalities. One of the key applications of 4-chloro-2,5-difluorobenzonitrile lies in medicinal chemistry, where halogenated nitriles are used as core structures in the design of enzyme inhibitors, receptor modulators, or other biologically active small molecules. The fluorine atoms are known to impact the metabolic stability, lipophilicity, and binding affinity of drug candidates. Moreover, the nitrile group can serve as a bioisostere for other polar functionalities, enhancing the compound's compatibility with biological targets. In agrochemical research, derivatives of 4-chloro-2,5-difluorobenzonitrile have been investigated as herbicide or pesticide precursors due to their ability to inhibit specific plant or insect enzymes. The rigidity and electronic properties of the aromatic core contribute to the selectivity and potency of such compounds in pest management strategies. This compound has also been explored in materials chemistry for its potential use in developing functional organic molecules, such as liquid crystals or polymers with tailored electronic properties. The presence of multiple electronegative atoms on the aromatic ring can influence molecular alignment and intermolecular interactions, which are critical in the design of high-performance organic materials. Synthesis of 4-chloro-2,5-difluorobenzonitrile is typically accomplished via halogenation of substituted benzonitriles or through a Sandmeyer-type reaction, starting from appropriately substituted aniline derivatives. Purification may involve recrystallization or chromatographic separation depending on the synthetic route and impurities formed. Safe handling practices for this compound include using appropriate personal protective equipment such as gloves and eye protection, as nitriles and halogenated aromatics can pose health hazards upon inhalation or skin contact. It should be stored in a tightly sealed container away from moisture and light to preserve its chemical stability. In conclusion, 4-chloro-2,5-difluorobenzonitrile is a valuable chemical intermediate that plays a significant role in modern synthetic chemistry. Its multifunctional nature enables a wide array of transformations, contributing to research and development across the pharmaceutical, agricultural, and materials sciences. References 2022. Synthesis of MRTX1719. Synfacts, 18(4). DOI: 10.1055/s-0041-1737934 |
| Market Analysis Reports |
| List of Reports Available for 4-Chloro-2,5-difluorobenzonitrile |