Online Database of Chemicals from Around the World
| Valiant Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (535) 610-1878 | |||
 |
jiangyanan@valiant-cn.com chenxiaowei@valiant-cn.com | |||
| Chemical manufacturer since 1992 | ||||
| chemBlink standard supplier since 2012 | ||||
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Aryl compounds >> Anilines |
|---|---|
| Name | 4-(Octadecyloxy)-1,3-benzenediamine |
| Molecular Structure | 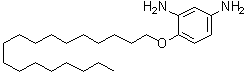 |
| Molecular Formula | C24H44N2O |
| Molecular Weight | 376.62 |
| CAS Registry Number | 155096-60-9 |
| SMILES | CCCCCCCCCCCCCCCCCCOC1=C(C=C(C=C1)N)N |
| Solubility | Insoluble (3.8E-5 g/L) (25 ºC), Calc.* |
|---|---|
| Density | 0.944±0.06 g/cm3 (20 ºC 760 Torr), Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software V11.02 (©1994-2017 ACD/Labs) |
|
4-(Octadecyloxy)-1,3-benzenediamine is an aromatic diamine compound with the molecular formula C24H43N2O. Structurally, it consists of a benzene ring substituted at the 1 and 3 positions with amino groups and at the 4 position with an octadecyloxy group, a long linear 18-carbon alkyl chain linked via an ether bond. This combination of a rigid aromatic core, nucleophilic amino groups, and a long hydrophobic chain imparts unique chemical and physical properties. The compound is typically a solid or waxy material at room temperature, soluble in organic solvents such as chloroform, toluene, and dichloromethane, but poorly soluble in water. The primary amino groups in 4-(Octadecyloxy)-1,3-benzenediamine make it highly reactive toward electrophiles, enabling its use as a building block in polymer synthesis and organic functionalization. It can participate in condensation reactions with acid chlorides, anhydrides, or aldehydes to form polyamides, polyimides, or Schiff base derivatives. The octadecyloxy substituent provides long-range hydrophobic character, improving solubility in nonpolar media and affecting the flexibility and self-assembly properties of resulting polymers. This compound is particularly useful in designing polymers and materials with tailored mechanical, thermal, and surface properties. The long alkyl chain can enhance hydrophobicity, reduce crystallinity, and impart flexibility to polymer backbones, while the aromatic diamine moiety contributes rigidity and the potential for hydrogen bonding or crosslinking. Such features are valuable in advanced material applications, including coatings, adhesives, and functionalized membranes. Synthetically, 4-(Octadecyloxy)-1,3-benzenediamine is typically prepared by nucleophilic aromatic substitution or Williamson ether synthesis, where an appropriate halogenated aromatic precursor reacts with octadecanol under basic conditions. Subsequent reduction or amination steps introduce the amino groups at the 1 and 3 positions. Purification methods include recrystallization or column chromatography to ensure the desired purity for further reactions. Overall, 4-(Octadecyloxy)-1,3-benzenediamine is a versatile aromatic diamine with both nucleophilic reactivity and hydrophobic character. Its structural combination of amino groups, an ether-linked long alkyl chain, and an aromatic ring makes it a valuable intermediate for the synthesis of specialty polymers, functional materials, and other organic derivatives where controlled solubility, flexibility, and reactivity are required. References 2024. Liquid crystal alignment agent, liquid crystal alignment film and liquid crystal element. CN Patent, 119264916. |
| Market Analysis Reports |
| List of Reports Available for 4-(Octadecyloxy)-1,3-benzenediamine |