Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt >> Metal sulfides and sulfates |
|---|---|
| Name | Pyridine sulfur trioxide |
| Synonyms | Sulfur trioxide pyridine complex |
| Molecular Structure | 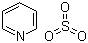 |
| Molecular Formula | C5H5N.SO3 |
| Molecular Weight | 159.16 |
| CAS Registry Number | 26412-87-3 |
| EC Number | 247-683-3 |
| SMILES | C1=CC=NC=C1.O=S(=O)=O |
| Melting point | 160 ºC (Expl.) |
|---|---|
| Solubility | water: solubility|reacts (Expl.) |
| Hazard Symbols |

 GHS05;GHS07 Danger Details
GHS05;GHS07 Danger Details | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H314-H315-H319 Details | ||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P260-P264-P264+P265-P270-P280-P301+P317-P301+P330+P331-P302+P352-P302+P361+P354-P304+P340-P305+P351+P338-P305+P354+P338-P316-P321-P330-P332+P317-P337+P317-P362+P364-P363-P405-P501 Details | ||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3261 | ||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||
|
Pyridine sulfur trioxide is a solid, isolable 1:1 complex formed between pyridine and sulfur trioxide that functions as a controlled source of SO3 in organic synthesis. The complex is produced by combining anhydrous pyridine with sulfur trioxide under strictly moisture-free conditions, yielding a white to off-white crystalline solid that is much easier to handle than liquid SO3. Its preparation and characterization were established in the mid-twentieth century during studies on sulfonation reagents and heterocycle–SO3 adducts, which were motivated by the need for safer alternatives to fuming sulfuric acid and oleum. The ability of pyridine to stabilize reactive electrophiles through Lewis basic coordination made it particularly suitable for forming a well-defined adduct with sulfur trioxide. As a result, pyridine sulfur trioxide became one of the earliest commercially practical solid SO3 complexes. The compound has played an important role in the development of modern sulfonation chemistry. Sulfonation reactions are central to the industrial preparation of dyes, detergents, pharmaceuticals, and agrochemicals, but historically relied on highly corrosive systems such as concentrated sulfuric acid or oleum. These traditional reagents pose handling difficulties and often lead to undesired side reactions or excessive degradation of sensitive substrates. The introduction of pyridine sulfur trioxide provided chemists with a standardized, easy-to-measure, and substantially safer method for delivering the sulfonating species. The complex releases SO3 under thermal or solvent-mediated activation and operates in a predictable, reproducible manner that enables selective modification of aromatic rings, heterocycles, aliphatic alcohols, and amines. One of the primary applications of pyridine sulfur trioxide is the sulfonation of alcohols to yield alkyl hydrogen sulfates. This transformation is widely used in the synthesis of surfactants and in the preparation of intermediates for industrial processes involving sulfated compounds. Because the reagent generates SO3 in a controlled fashion, it allows direct conversion of alcohols to sulfate esters under milder conditions than those required for free sulfur trioxide or oleum. In addition, the reaction can be tuned by choice of solvent and temperature, enabling selective formation of mono- or bis-sulfated products depending on substrate and conditions. The complex is also widely employed in the preparation of amine sulfonates and quaternary ammonium sulfonates. These products find use as zwitterionic surfactants, phase-transfer agents, and components of functional materials. Pyridine sulfur trioxide is effective in converting primary and secondary amines into their corresponding sulfamates, which are valuable intermediates in medicinal chemistry. Because the reagent minimizes over-sulfonation and reduces formation of polysulfonated by-products, it has been adopted in synthetic routes involving substrates that are sensitive to strong acids or high reaction temperatures. In aromatic sulfonation, pyridine sulfur trioxide provides enhanced selectivity relative to conventional sulfuric acid-based systems. The reagent allows access to arylsulfonic acids and sulfonyl derivatives without the excessive oxidation or rearrangement often observed with harsher reagents. It has been used in the preparation of substituted benzenesulfonic acids, naphthalenesulfonic acids, and heteroaromatic sulfonates, which serve as key intermediates in dye manufacture, polymer chemistry, and pharmaceutical synthesis. In particular, the reagent has proven effective in sulfonating electron-rich aromatic systems that are prone to decomposition under strongly acidic conditions. The role of pyridine sulfur trioxide in peptide and carbohydrate chemistry is also well documented. In carbohydrate functionalization, it can selectively sulfate hydroxyl groups to afford sulfated sugars used in biochemical studies and therapeutic development. In peptide modification, the reagent is applied in the synthesis of sulfated amino acids and peptide fragments, which are important in studies of signaling pathways, enzyme recognition, and receptor binding. The ability of the complex to operate in polar aprotic solvents facilitates functionalization of biomolecules that require controlled conditions to preserve structural integrity. Overall, pyridine sulfur trioxide represents a cornerstone reagent for controlled sulfonation across a broad range of substrates. Its discovery marked a significant improvement in the safety and versatility of SO3 delivery. Its applications continue to influence industrial and laboratory processes in organic synthesis, materials chemistry, and biochemistry. References Gilbert EE (1962) The reactions of sulfur trioxide, and of its adducts, with organic compounds. Chemical Reviews 62 549–589 DOI: 10.1021/cr60220a003 Alshehri JA, Jones AM (2024) Chemical approaches to the sulfation of small molecules: current progress and future directions. Essays in Biochemistry 68 449–466 DOI: 10.1042/EBC20240001 |
| Market Analysis Reports |
| List of Reports Available for Pyridine sulfur trioxide |