Online Database of Chemicals from Around the World
| Wuhan Lwax Pharma Tech Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 1313 567 7601 | |||
 |
hanna@whlwax.com | |||
| Chemical distributor since 2021 | ||||
| chemBlink standard supplier since 2021 | ||||
| Wuhan Xiju Biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13043116031 | |||
 |
Fan@wh-xiju.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2021 | ||||
| chemBlink standard supplier since 2021 | ||||
| Hongyan Pharmaceutical Technology (Wuhan) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 19565688180 | |||
 |
alice@whhoyan.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2017 | ||||
| chemBlink standard supplier since 2023 | ||||
| Hubei Asli New Materials Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13135651187 | |||
 |
admin@aslbmkpmk.com | |||
 |
Skype Chat | |||
 |
WeChat: Hubei asli new materials technology co.,ltd | |||
 |
WhatsApp: +86-18031176193 | |||
| Chemical distributor since 2022 | ||||
| chemBlink standard supplier since 2023 | ||||
| Wuhan Ruichi Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13545065237 | |||
 |
admin@whrchem.com | |||
 |
WhatsApp: +8613545065237 | |||
| Chemical manufacturer since 2021 | ||||
| chemBlink standard supplier since 2023 | ||||
| Classification | Flavors and spices >> Synthetic spice >> Carboxylic acid and ester perfume >> Aromatic carboxylic acid ester |
|---|---|
| Name | Ethyl 3-oxo-2-phenylbutanoate |
| Synonyms | etyl-2-fenyl-3-oxobutano�t |
| Molecular Structure | 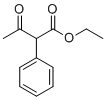 |
| Molecular Formula | C12H14O3 |
| Molecular Weight | 206.24 |
| CAS Registry Number | 5413-05-8 |
| EC Number | 226-500-0 |
| SMILES | CCOC(=O)C(C1=CC=CC=C1)C(=O)C |
| Solubility | 2437 mg/L (25 ºC water) |
|---|---|
| Density | 1.1±0.1 g/cm3, Calc.* |
| Index of Refraction | 1.504, Calc.* |
| Melting point | 55.74 ºC |
| Boiling Point | 290.51 ºC, 281.6±20.0 ºC (760 mmHg), Calc.* |
| Flash Point | 119.2±21.8 ºC, Calc.* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
Ethyl 3-oxo-2-phenylbutanoate, also known as ethyl phenylglyoxylate, was discovered through organic synthesis endeavors in the field of medicinal and synthetic chemistry. Its synthesis involves the reaction of ethyl acetoacetate with benzaldehyde, leading to the formation of the desired compound. This chemical compound was first reported in scientific literature in the early 20th century, and its structure was confirmed through spectroscopic analysis and chemical characterization techniques. The discovery of Ethyl 3-oxo-2-phenylbutanoate opened up avenues for research into its chemical properties and potential applications in various fields. Ethyl 3-oxo-2-phenylbutanoate serves as a valuable intermediate in the synthesis of pharmaceutical compounds. It participates in diverse chemical transformations, including condensation reactions and reduction reactions, to form complex organic molecules with potential pharmacological activities. It is commonly used in the production of pharmaceuticals such as analgesics, antipyretics, and anti-inflammatory drugs. This compound finds applications in the flavor and fragrance industry as a flavoring agent and fragrance ingredient. Its pleasant aroma contributes to the formulation of perfumes, colognes, and scented products. It provides fruity, floral, or sweet notes to fragrances and enhances their olfactory appeal and complexity. Ethyl 3-oxo-2-phenylbutanoate is utilized as a building block in organic synthesis for the preparation of various fine chemicals and specialty compounds. Organic chemists employ it as a precursor for the synthesis of substituted ketones, esters, and carboxylic acids through diverse chemical reactions. Its versatility in chemical transformations allows for the creation of structurally diverse molecules for applications in materials science, agrochemicals, and other fields. Researchers utilize Ethyl 3-oxo-2-phenylbutanoate in laboratory experiments and chemical research for studying reaction mechanisms, exploring new synthetic methodologies, and developing novel chemical processes. Its unique chemical structure and reactivity make it a valuable tool for investigating organic reactions and designing efficient synthetic routes for target molecules. Ethyl 3-oxo-2-phenylbutanoate may find use as a food additive or flavor enhancer in the food industry. Its fruity and sweet aroma can enhance the flavor profile of food products such as beverages, confectioneries, and baked goods. It is approved for use in certain food applications where it imparts desirable sensory attributes without posing health risks to consumers. References 2004. Approaches for introducing high molecular diversity in scaffolds: Fast parallel synthesis of highly substituted 1H-quinolin-4-one libraries. Molecular Diversity, 8(4). DOI: 10.1023/b:modi.0000047511.72472.ea 2014. Bromodeacylation of β-Oxo Esters. Science of Synthesis. URL: https://science-of-synthesis.thieme.com/app/text/?id=SD-135-00038 2020. Semi-Industrial Fluorination of β-Keto Esters with SF4: Safety vs Efficacy. Synlett, 31(3). DOI: 10.1055/s-0037-1610744 |
| Market Analysis Reports |
| List of Reports Available for Ethyl 3-oxo-2-phenylbutanoate |