Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Classification | Organic raw materials >> Hydrocarbon compounds and their derivatives >> Aromatic hydrocarbon |
|---|---|
| Name | 2-Methylresorcinol |
| Synonyms | 2-Methyl-1,3-benzenediol; 2,6-Dihydroxytoluene |
| Molecular Structure | 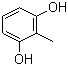 |
| Molecular Formula | C7H8O2 |
| Molecular Weight | 124.14 |
| CAS Registry Number | 608-25-3 |
| EC Number | 210-155-8 |
| SMILES | CC1=C(C=CC=C1O)O |
| Melting point | 114-120 ºC (Expl.) |
|---|---|
| Boiling point | 264 ºC (Expl.) |
| Flash point | 135 ºC (closed cup) (Expl.) |
| Hazard Symbols |



 GHS05;GHS06;GHS07;GHS09 Danger Details
GHS05;GHS06;GHS07;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H301-H315-H317-H318-H319-H335-H400-H410 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P270-P271-P272-P273-P280-P301+P316-P302+P352-P304+P340-P305+P351+P338-P305+P354+P338-P317-P319-P321-P330-P332+P317-P333+P317-P337+P317-P362+P364-P391-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||
|
2-Methylresorcinol, also known by its IUPAC name 2-methylbenzene-1,3-diol, is an aromatic organic compound consisting of a benzene ring substituted with two hydroxyl groups in the meta positions (1 and 3) and a methyl group at the 2-position. It belongs to the class of resorcinol derivatives, which are well-studied phenolic compounds widely used in chemical synthesis, cosmetics, and pharmaceuticals. The molecular formula of 2-methylresorcinol is C7H8O2. The compound is structurally related to resorcinol (benzene-1,3-diol), differing by the presence of a methyl group on the aromatic ring. This methyl group modifies the electronic properties and reactivity of the molecule, potentially affecting its behavior in chemical reactions and biological systems. 2-Methylresorcinol is typically obtained through synthetic organic methods, such as the methylation of resorcinol under controlled conditions. 2-Methylresorcinol has found applications primarily in the cosmetic and hair dye industries. It is used as an intermediate in the synthesis of hair colorants, especially oxidative hair dyes, where it functions as a coupling agent. In these systems, primary intermediates such as p-phenylenediamine derivatives react with coupling agents like 2-methylresorcinol in the presence of an oxidizing agent (usually hydrogen peroxide) to produce colored dye molecules. The position of the hydroxyl groups and the methyl substituent contributes to the development of specific shades and enhances the color intensity and fastness of the final product. The compound’s phenolic groups allow it to participate in electrophilic substitution reactions, oxidation-reduction processes, and hydrogen bonding, making it a useful building block in organic synthesis. Beyond its role in hair dyes, 2-methylresorcinol has also been studied in research settings as a model compound for understanding the behavior of substituted phenols in both biological and environmental systems. In analytical chemistry and toxicology, phenolic compounds such as 2-methylresorcinol are evaluated for their potential effects on human health and the environment. While resorcinol derivatives are generally considered safe at regulated concentrations, safety assessments are routinely conducted to determine acceptable exposure levels in consumer products. In hair dye formulations, the use of 2-methylresorcinol is subject to regulation in many regions, and it is included in various safety databases and cosmetic ingredient inventories. The compound can be characterized using standard spectroscopic and chromatographic techniques. In infrared spectroscopy, it shows characteristic absorptions for hydroxyl (–OH) and aromatic C–H bonds. Nuclear magnetic resonance (NMR) spectroscopy provides information about the chemical environment of the aromatic protons and the methyl group. Mass spectrometry can be used to determine the molecular weight and fragmentation pattern, confirming the identity of the compound. The chemical is usually handled in its crystalline or solid form and is soluble in organic solvents such as ethanol and ether, as well as slightly soluble in water. Its physical and chemical properties make it suitable for use in formulations that require precise control of reactivity and solubility. In summary, 2-methylresorcinol is a methylated dihydroxybenzene used primarily as a coupling component in oxidative hair dye formulations. Its structural features and reactivity make it useful in cosmetic chemistry and organic synthesis. While not used therapeutically, it plays a key role in consumer products requiring stable and effective color-producing agents. References 2024. Chemoenzymatic total synthesis of sorbicillactone A. Communications Chemistry, 7(1). DOI: 10.1038/s42004-024-01126-1 2023. Secondary Metabolites and Biological Activity of Endophytic Fungus Aspergillus terreus from Plantago asiatica. Chemistry of Natural Compounds, 59(4). DOI: 10.1007/s10600-023-04113-5 2022. Greener Syntheses of Coumarin Derivatives Using Magnetic Nanocatalysts: Recent Advances. Topics in current chemistry (Cham), 380(6). DOI: 10.1007/s41061-022-00407-4 |
| Market Analysis Reports |
| List of Reports Available for 2-Methylresorcinol |