Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Organic raw materials >> Hydrocarbon compounds and their derivatives >> Hydrocarbon halide |
|---|---|
| Name | 2-Chloropropane |
| Synonyms | Isopropyl chloride |
| Molecular Structure | 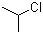 |
| Molecular Formula | C3H7Cl |
| Molecular Weight | 78.54 |
| CAS Registry Number | 75-29-6 |
| EC Number | 200-858-8 |
| SMILES | CC(C)Cl |
| Density | 0.9±0.1 g/cm3 Calc.*, 0.859 g/mL (Expl.) |
|---|---|
| Melting point | -118 ºC (Expl.) |
| Boiling point | 37.3±8.0 ºC 760 mmHg (Calc.)*, 34 - 36 ºC (Expl.) |
| Flash point | -35.0 ºC (Calc.)*, -21 ºC (Expl.) |
| Solubility | water 3.1 g/L (20 ºC) (Expl.) |
| Index of refraction | 1.379 (Calc.)*, 1.378 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS02;GHS07 DangerGHS02 Details
GHS02;GHS07 DangerGHS02 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H225-H302-H312-H332 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P261-P264-P270-P271-P280-P301+P317-P302+P352-P303+P361+P353-P304+P340-P317-P321-P330-P362+P364-P370+P378-P403+P235-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 2356 | ||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||
|
2-Chloropropane, also known as isopropyl chloride, is a secondary alkyl halide with the molecular formula C3H7Cl. It is a colorless, flammable liquid with a characteristic ether-like odor. As a haloalkane, it contains a chlorine atom attached to the secondary carbon of a propyl group, which gives it distinct chemical reactivity, including nucleophilic substitution and elimination reactions. Its secondary carbon center affects its steric accessibility and influences the reaction pathways it undergoes compared with primary or tertiary halides. The discovery of 2-chloropropane is linked to early studies of alcohol halogenation. It was initially synthesized by reacting isopropanol with hydrochloric acid under acidic conditions, where the hydroxyl group is substituted by a chlorine atom. Later improvements included the use of thionyl chloride or phosphorus pentachloride as chlorinating agents to provide higher yields and purities while minimizing side reactions. These methods established practical procedures for both laboratory-scale and industrial production of 2-chloropropane. 2-Chloropropane finds extensive use as an intermediate in organic synthesis. Its secondary chloride group allows it to act as an alkylating agent for nucleophiles, enabling the construction of isopropyl-substituted molecules. In pharmaceutical chemistry, it is utilized to synthesize intermediates for drugs, where isopropyl substitution can modify biological activity, solubility, and metabolic stability. It is also used in the preparation of agrochemicals, providing a route to herbicides, insecticides, and fungicides that contain the isopropyl group. In polymer and materials chemistry, 2-chloropropane can be used to introduce branching or functional groups into polymer backbones through substitution reactions. This modification can improve polymer properties such as solubility, flexibility, and reactivity for subsequent chemical transformations. In research laboratories, 2-chloropropane serves as a model compound for studying SN1 and SN2 reaction mechanisms, as its secondary carbon allows for both substitution and elimination pathways depending on reaction conditions. Mechanistically, 2-chloropropane undergoes nucleophilic substitution through both SN2 and SN1 pathways. Steric hindrance at the secondary carbon makes SN2 reactions slower than for primary chlorides, particularly with bulky nucleophiles, while polar protic solvents and weak nucleophiles can favor carbocation formation and SN1 reactions. Under basic conditions, 2-chloropropane can also undergo dehydrohalogenation to form propene. This combination of reactivity makes it a valuable reagent for exploring organic reaction mechanisms. Physically, 2-chloropropane is slightly soluble in water and miscible with many organic solvents such as alcohols, ethers, and hydrocarbons. It is highly flammable and volatile, requiring careful storage in cool, well-ventilated areas away from sources of ignition. Safety precautions are important because 2-chloropropane can be harmful if inhaled, ingested, or absorbed through the skin. Proper personal protective equipment and adherence to disposal protocols are essential when handling this chemical. Overall, 2-chloropropane is a versatile secondary alkyl halide with important applications in organic synthesis, pharmaceuticals, agrochemicals, and polymer chemistry. Its ability to undergo substitution and elimination reactions, along with its utility in introducing isopropyl groups, makes it an essential reagent in both research and industrial contexts. References 2024. Synthesis and Biological Evaluation of 1,3,5-Dithiazinanes: Synthesis, Stereochemistry and Applications. S-Heterocycles. DOI: 10.1007/978-981-97-4308-7_5 2023. Biological potential of vinyl/allyl substituted imidazole-based N-heterocyclic carbene adducts: synthesis, spectral and X-ray crystallographic structural characteristics. Chemical Papers, 77(12). DOI: 10.1007/s11696-023-03126-2 2022. Deoxygenative alkylation of tertiary amides using alkyl iodides under visible light. Science China Chemistry, 65(9). DOI: 10.1007/s11426-022-1331-y |
| Market Analysis Reports |
| List of Reports Available for 2-Chloropropane |