Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Classification | Inorganic chemical industry >> Inorganic salt >> Metal halides and halides >> Metal chlorides and salts |
|---|---|
| Name | Palladium chloride |
| Synonyms | Palladium (II) chloride |
| Molecular Structure | 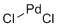 |
| Molecular Formula | PdCl2 |
| Molecular Weight | 177.33 |
| CAS Registry Number | 7647-10-1 |
| EC Number | 231-596-2 |
| SMILES | Cl[Pd]Cl |
| Density | 4 g/mL (Expl.) |
|---|---|
| Melting point | 678-680 ºC (Expl.) |
| Solubility | soluble (water 100 ºC, HCL, acetone) (Expl.) |
| Hazard Symbols |



 GHS05;GHS06;GHS07;GHS09 Danger Details
GHS05;GHS06;GHS07;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H290-H301-H302-H315-H317-H318-H319-H335-H400-H410 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P234-P261-P264-P264+P265-P270-P271-P272-P273-P280-P301+P316-P301+P317-P302+P352-P304+P340-P305+P351+P338-P305+P354+P338-P317-P319-P321-P330-P332+P317-P333+P317-P337+P317-P362+P364-P390-P391-P403+P233-P405-P406-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 1789 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Palladium chloride (PdCl2) is a widely used and well-characterized chemical compound of palladium, a transition metal with significant industrial and chemical importance. Palladium chloride exists in two primary forms: the anhydrous PdCl2 and the hydrated PdCl2·2H2O, with the anhydrous form being the most commonly referenced in scientific literature. It is a yellowish-brown solid that is soluble in water and alcohols, and it is primarily used as a catalyst in various chemical reactions, particularly in the field of organic synthesis. The discovery of palladium chloride can be traced back to the early 19th century, following the discovery of palladium itself. Palladium was first identified in 1803 by the English chemist William Hyde Wollaston, who isolated it from platinum ores. Palladium chloride was later prepared by dissolving palladium metal in aqua regia (a mixture of hydrochloric and nitric acids) and then evaporating the solution, leading to the formation of PdCl2. The compound has since been extensively studied due to its unique chemical properties and its widespread use in catalytic processes. Palladium chloride is most notably employed as a catalyst in a variety of important organic reactions. One of its key applications is in the catalysis of hydrogenation reactions, where it facilitates the addition of hydrogen to alkenes, alkynes, and other unsaturated compounds. This reaction is fundamental in the production of a wide range of chemicals, including pharmaceuticals, plastics, and food products. Palladium chloride is particularly effective in hydrogenation reactions because of its ability to adsorb hydrogen molecules onto its surface, which aids in the efficient conversion of unsaturated substrates into saturated ones. Another important application of palladium chloride is in the realm of cross-coupling reactions, such as the Suzuki-Miyaura coupling, the Heck reaction, and the Stille coupling. These reactions involve the formation of carbon-carbon bonds and are widely used in the synthesis of complex organic molecules, including pharmaceuticals, agrochemicals, and materials. Palladium chloride is often used as a precursor to generate active palladium species that are essential for these reactions. Its ability to catalyze oxidative addition and reductive elimination steps makes it a versatile catalyst in these processes. Palladium chloride also plays a role in the formation of organopalladium complexes, which are widely used in various catalytic cycles. By coordinating with ligands such as phosphines, phosphites, and arsines, PdCl2 can form highly active catalytic species that are used in a range of reactions, including cross-coupling, polymerization, and cyclization reactions. In these processes, PdCl2 is often used in conjunction with other reagents or solvents to optimize reaction conditions and increase yields. In addition to its use in catalysis, palladium chloride has been employed in other areas, such as in the production of palladium metal through reduction processes, and as a precursor for the synthesis of other palladium compounds. It is also used in electroplating applications, where it is deposited onto substrates to create thin palladium coatings. These coatings are used in a variety of industries, including electronics and jewelry, due to palladium's corrosion resistance and electrical conductivity. Palladium chloride has also found applications in the field of analytical chemistry. It is used as a reagent in the detection and quantification of various substances, including halides, sulfur compounds, and other metal ions. Its ability to form complexes with a wide range of chemical species makes it a valuable tool for chemical analysis. In summary, palladium chloride is an important chemical compound with diverse applications in industrial chemistry, organic synthesis, and analytical chemistry. Its discovery dates back to the early 19th century, and it has since become a key catalyst in a variety of reactions, particularly those involving hydrogenation and cross-coupling. The compound's ability to form stable organopalladium complexes has made it invaluable in the development of efficient catalytic processes, and it continues to be widely used in the production of pharmaceuticals, agrochemicals, and materials. References 2024. Mechanistic Investigation of Dimethyl Carbonate Synthesis Over Palladium Chloride Catalyst. Catalysis Letters. DOI: 10.1007/s10562-024-04855-z 2008. Palladium-catalyzed decarboxylative cross-coupling reaction of cinnamic acid with aryl iodide. Organic & Biomolecular Chemistry, 7(4). DOI: 10.1039/b821870f 2008. Tandem addition-cyclization reactions of 2-alkynylbenzenamines with isocyanates catalyzed by PdCl2. Organic & Biomolecular Chemistry, 6(24). DOI: 10.1039/b812015c |
| Market Analysis Reports |
| List of Reports Available for Palladium chloride |