Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Classification | Chemical reagent >> Organic reagent >> Acid halide |
|---|---|
| Name | Chloroacetyl chloride |
| Synonyms | Chloroacetic ahloride; Chloroacetylchloride; Monochloroacetyl chloride |
| Molecular Structure | 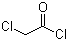 |
| Molecular Formula | C2H2Cl2O |
| Molecular Weight | 112.94 |
| CAS Registry Number | 79-04-9 |
| EC Number | 201-171-6 |
| SMILES | C(C(=O)Cl)Cl |
| Density | 1.4±0.1 g/cm3, Calc.*, 1.418 g/mL (Expl.) |
|---|---|
| Melting point | -22 ºC (Expl.) |
| Index of Refraction | 1.43, Calc.*, 1.453 (Expl.) |
| Boiling Point | 106.0 ºC (760 mmHg), Calc.*, 105-106 ºC (Expl.) |
| Flash Point | 34.0±20.2 ºC, Calc.*, 100 ºC (Expl.) |
| Water solubility | reacts |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |



 GHS06;GHS08;GHS05;GHS09 Danger Details
GHS06;GHS08;GHS05;GHS09 Danger Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H290-H301-H310-H311-H314-H318-H331-H372-H400-H410 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P234-P260-P261-P262-P264-P264+P265-P270-P271-P273-P280-P301+P316-P301+P330+P331-P302+P352-P302+P361+P354-P304+P340-P305+P354+P338-P316-P317-P319-P321-P330-P361+P364-P363-P390-P391-P403+P233-P405-P406-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 1752 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Chloroacetyl chloride (C2H3ClO) is an organic compound that consists of a chloro group (Cl) attached to an acetyl group (COCH3). It is a colorless, corrosive liquid with a pungent odor and is highly reactive, particularly with water, which it hydrolyzes to form acetic acid and hydrogen chloride. Chloroacetyl chloride is used in a variety of chemical syntheses and industrial applications. The discovery of chloroacetyl chloride is linked to the development of acyl chloride chemistry in the late 19th and early 20th centuries. Acyl chlorides, including chloroacetyl chloride, were found to be useful intermediates in organic synthesis due to their ability to react with nucleophiles, such as amines, alcohols, and thiols, forming derivatives like amides, esters, and thioesters. This reactivity made chloroacetyl chloride a valuable reagent in both laboratory and industrial settings. One of the primary applications of chloroacetyl chloride is in the synthesis of pharmaceuticals. It is used to manufacture certain drugs, including those used in cancer treatment, by acting as an acylating agent to introduce the acetyl group into organic molecules. For example, chloroacetyl chloride can be used in the preparation of acetylated derivatives of amino acids and peptides, which are important components of many pharmaceutical compounds. Chloroacetyl chloride is also utilized in the production of agrochemicals, where it is involved in the synthesis of herbicides and insecticides. Its ability to react with various compounds makes it an effective intermediate in the formation of complex organic molecules used for pest control. Furthermore, it is employed in the manufacture of specialty chemicals, such as plasticizers and surfactants, which are used in a wide range of applications from coatings to detergents. In the polymer industry, chloroacetyl chloride is used as a starting material for the synthesis of various polymers, including those used in coatings, adhesives, and plastics. It can be used to modify polymer chains, enhancing the properties of the final product. Additionally, it is sometimes used as a reagent in the preparation of functionalized materials with specific chemical properties. Chloroacetyl chloride is highly reactive and must be handled with care. It is a strong irritant to the skin, eyes, and respiratory system, and exposure can cause severe chemical burns. It should be stored in tightly sealed containers in cool, dry conditions, away from moisture and incompatible materials. Safety precautions, including wearing appropriate protective gear such as gloves, goggles, and a lab coat, are necessary when handling this compound. In summary, chloroacetyl chloride is an important chemical intermediate used in a wide range of applications, including pharmaceuticals, agrochemicals, and polymer production. Its reactivity makes it a valuable reagent in organic synthesis, enabling the preparation of a variety of functionalized compounds. However, its corrosive nature requires careful handling and adherence to safety protocols. References 2013. The synthesis and biological testing of bacilysin analogues. Amino Acids, 45(5). DOI: 10.1007/s00726-013-1571-4 2017. Synthesis, characterization, biological evaluation and molecular docking studies of 2-(1H-benzo[d]imidazol-2-ylthio)-N-(substituted 4-oxothiazolidin-3-yl) acetamides. Chemistry Central Journal, 11(1). DOI: 10.1186/s13065-017-0312-2 2018. Trace level determination of chloroacetyl chloride and degradation products by derivatization gas chromatography. Journal of Pharmaceutical and Biomedical Analysis, 149. DOI: 10.1016/j.jpba.2017.09.026 |
| Market Analysis Reports |
| List of Reports Available for Chloroacetyl chloride |