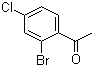
2'-Bromo-4'-chloroacetophenone is a halogenated aromatic ketone in which a benzene ring is substituted with a bromine atom at the ortho position and a chlorine atom at the para position relative to the acetyl group. The compound belongs to the acetophenone family, which consists of aromatic ketones widely used as intermediates in organic synthesis. The presence of halogen substituents modulates the electronic properties of the aromatic ring, affecting reactivity in electrophilic and nucleophilic aromatic substitution reactions. The combination of bromine, chlorine, and the carbonyl functionality makes 2'-bromo-4'-chloroacetophenone a versatile building block for chemical transformations in both medicinal and materials chemistry.
The synthesis of 2'-bromo-4'-chloroacetophenone generally involves halogenation of 4-chloroacetophenone or bromination of 2-bromo-4-chloroacetophenone precursors under controlled conditions. Careful selection of reagents, temperature, and solvent is essential to achieve selective halogenation without over-substitution or oxidation of the ketone group. The compound is typically obtained as a crystalline or oily solid and can be purified through recrystallization or column chromatography. Its stability under ambient conditions facilitates storage and use in multistep synthetic sequences.
In organic synthesis, 2'-bromo-4'-chloroacetophenone is primarily used as an intermediate for the preparation of substituted aromatic and heteroaromatic compounds. The bromine atom can participate in palladium-catalyzed cross-coupling reactions, such as Suzuki, Heck, or Sonogashira couplings, enabling the introduction of aryl, vinyl, or alkynyl groups at a defined position. The chlorine substituent provides additional opportunities for nucleophilic aromatic substitution, while the ketone group serves as a site for condensation, reduction, or enolate chemistry. This combination of reactive sites allows construction of complex molecules with controlled substitution patterns and electronic properties.
In medicinal chemistry, derivatives of 2'-bromo-4'-chloroacetophenone have been used as intermediates in the synthesis of pharmacologically active molecules. The halogenated aromatic ketone scaffold is valuable for designing enzyme inhibitors, receptor ligands, and other bioactive compounds. The ketone group allows introduction of various functional groups, while the halogens influence molecular recognition, lipophilicity, and metabolic stability. Such derivatives have been incorporated into structure–activity relationship studies to optimize biological properties and target specificity.
The compound has also found applications in materials chemistry and chemical methodology research. Halogenated acetophenones are useful in the preparation of dyes, fluorescent probes, and functional polymers, where the halogen atoms can modulate electronic and photophysical properties. The ketone functionality allows for covalent attachment or further derivatization, enabling the design of functional materials with desired optical, electronic, or structural characteristics. Additionally, 2'-bromo-4'-chloroacetophenone serves as a model substrate in mechanistic studies of halogenated ketone reactivity, providing insight into regioselectivity, electronic effects, and reaction optimization.
Physically, 2'-bromo-4'-chloroacetophenone is a stable solid with moderate solubility in polar organic solvents such as dichloromethane, ethanol, and acetone. Proper storage, protected from moisture and strong nucleophiles, ensures retention of its chemical integrity for synthetic applications. Its combination of stability, reactivity, and dual halogenation makes it a versatile intermediate for the preparation of structurally diverse aromatic compounds.
Overall, 2'-bromo-4'-chloroacetophenone is an important halogenated aromatic ketone used extensively as a synthetic intermediate. Its bromine and chlorine substituents, together with the carbonyl group, provide multiple avenues for selective transformations, enabling the construction of complex molecules for pharmaceutical, agrochemical, and materials research.
References
2'-Bromo-4'-chloroacetophenone|2008. Growth and characterization studies of 2-bromo-4'-chloroacetophenone (BCAP) crystals. Journal of Thermal Analysis and Calorimetry, 94(2).
DOI: 10.1007/s10973-008-9188-7
|
















 GHS07 Warning Details
GHS07 Warning Details