Online Database of Chemicals from Around the World
| Alfa Chemistry | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (201) 478-8534 | |||
 |
inquiry@alfa-chemistry.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2012 | ||||
| Hubei Innerse Biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (027) 8818-9299 | |||
 |
sales@innersebio.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink standard supplier since 2025 | ||||
| AnaSpec, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (800) 452-5530 | |||
 |
service@anaspec.com | |||
| Chemical manufacturer | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Indoles |
|---|---|
| Name | 1,1'-Dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate |
| Synonyms | DiIC16(3) perchlorate |
| Molecular Structure | 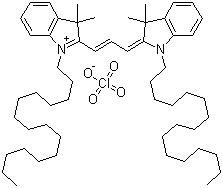 |
| Molecular Formula | C55H89ClN2O4 |
| Molecular Weight | 877.76 |
| CAS Registry Number | 84109-11-5 |
| SMILES | CCCCCCCCCCCCCCCCN1C2=CC=CC=C2C(C1=CC=CC3=[N+](C4=CC=CC=C4C3(C)C)CCCCCCCCCCCCCCCC)(C)C.[O-]Cl(=O)(=O)=O |
|
1,1'-Dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate is a carbocyanine dye belonging to the family of cationic polymethine dyes. It consists of two indole-derived heterocyclic units linked by a conjugated polymethine chain, with each nitrogen atom substituted by a hexadecyl (C16) alkyl group. The indole rings carry geminal dimethyl substituents at the 3-positions, while the overall molecule bears a positive charge that is balanced by a perchlorate counterion. The extended conjugated system is responsible for its strong absorption and fluorescence properties, typically in the red to near-infrared region of the electromagnetic spectrum. The compound generally appears as a deeply colored solid and shows high solubility in organic solvents such as chloroform, dichloromethane, and alcohols, while being practically insoluble in water due to its long hydrophobic alkyl chains. This compound is primarily used as a fluorescent probe and membrane-labeling dye. The presence of long hexadecyl chains imparts strong lipophilicity, enabling efficient incorporation into lipid bilayers, micelles, and other hydrophobic environments. As a result, it has been widely applied in studies of biological membranes, vesicle dynamics, and lipid organization. Its fluorescence properties allow visualization and tracking of membrane-associated processes using fluorescence microscopy and spectroscopy. The perchlorate salt form is commonly employed because it provides good stability and consistent optical behavior in solution. 1,1'-Dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate is also utilized in photophysical and materials research. Carbocyanine dyes of this type are investigated for their strong molar absorptivity, narrow absorption bands, and efficient fluorescence emission. These characteristics make them suitable for use in optical sensors, energy-transfer studies, and model systems for investigating excited-state dynamics. In some cases, such dyes are incorporated into thin films or nanostructured materials to explore charge transport and light-harvesting behavior. The synthesis of this compound typically involves the preparation of alkylated indole precursors, followed by condensation to form the polymethine bridge and subsequent quaternization to generate the cationic indocarbocyanine structure. Final isolation as the perchlorate salt yields a stable, well-defined dye suitable for experimental use. Due to its strong coloration and sensitivity to light, the compound is usually handled under subdued lighting and stored in tightly sealed containers to prevent photodegradation. Overall, 1,1'-dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate is a well-established carbocyanine dye whose combination of strong fluorescence, extended conjugation, and pronounced lipophilicity underpins its widespread use in membrane studies, fluorescence imaging, and photophysical research. References 2024. Lipid aggregates for intraarticular administration. WO Patent. URL: WO-2025017206-A1 2024. Natural lipid particle formulations for agricultural applications. WO Patent. URL: WO-2024168193-A2 |
| Market Analysis Reports |
| List of Reports Available for 1,1'-Dihexadecyl-3,3,3',3'-tetramethylindocarbocyanine perchlorate |