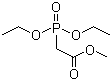
Methyl diethylphosphonoacetate is an organophosphorus compound widely used as a synthetic reagent in organic chemistry. Its molecular formula is C7H15O5P, and its structure consists of a phosphonate group bearing two ethoxy substituents and linked through a methylene group to a methyl ester of acetic acid. This combination of a stabilized phosphonate and an ester functionality gives the molecule distinctive reactivity that is exploited in carbon–carbon bond-forming reactions. Under ambient conditions, methyl diethylphosphonoacetate is typically a colorless to pale yellow liquid with good solubility in common organic solvents and limited solubility in water.
The compound is best known for its role in the Horner–Wadsworth–Emmons reaction, a variant of the Wittig olefination. In this reaction, methyl diethylphosphonoacetate is deprotonated at the methylene position adjacent to the phosphonate group to form a stabilized phosphonate carbanion. This anion reacts with aldehydes or ketones to form carbon–carbon double bonds, yielding α,β-unsaturated esters with high selectivity. Because the phosphonate group stabilizes the carbanion, reactions involving this reagent generally proceed under milder conditions and with better control over stereochemistry than reactions using unstabilized phosphonium ylides.
The development and adoption of phosphonate reagents such as methyl diethylphosphonoacetate marked an important advance in synthetic organic chemistry. These reagents enabled more predictable synthesis of conjugated esters, which are key intermediates in pharmaceuticals, agrochemicals, fragrances, and natural product synthesis. The ester group present in the product alkenes also provides a versatile handle for further functionalization, including hydrolysis, reduction, or substitution reactions.
Methyl diethylphosphonoacetate is commonly prepared by esterification of diethyl phosphonoacetic acid or by related phosphorylation and alkylation strategies involving phosphite esters. In laboratory practice, it is handled as a moisture-stable liquid, although strong bases are required to generate the reactive anion used in olefination reactions. Typical bases include alkoxides or metal amides, chosen according to the substrate and desired reaction conditions.
Beyond olefination chemistry, this compound has been employed as a building block in the synthesis of heterocycles and biologically active molecules. The phosphonate group can be selectively transformed or removed after carbon–carbon bond formation, allowing chemists to use it as a temporary activating and directing group. This strategic flexibility has made methyl diethylphosphonoacetate a standard reagent in academic and industrial synthetic laboratories.
Overall, methyl diethylphosphonoacetate is a well-established phosphonate reagent whose importance lies in its reliable reactivity and broad applicability in carbon–carbon bond formation. Its role in the Horner–Wadsworth–Emmons reaction and related transformations has made it a cornerstone reagent for constructing unsaturated esters and complex molecular frameworks in modern organic synthesis.
References
2021. Applications of the Horner-Wadsworth-Emmons Olefination in Modern Natural Product Synthesis. Synthesis, 53(19).
DOI: 10.1055/a-1493-6331
2017. Structure-based design and synthesis of acyclic and substituted heterocyclic phosphonates linearly linked to thiazolobenzimidazoles as potent hydrophilic antineoplastic agents. Chemical Papers, 71(10).
DOI: 10.1007/s11696-017-0190-z
|


















 GHS07 Warning Details
GHS07 Warning Details