Online Database of Chemicals from Around the World
| Reddy N Reddy Pharmaceuticals | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (40) 2307-5686 +91 98480-96759 +91 98481-96759 | |||
 |
info@reddynreddypharma.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Rhodia (shanghai) International Trading Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 2408-9317 +86 13601753458 | |||
 |
hank.xue@ap.rhodia.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Wuhan Kemi-works Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (27) 8573-6489 | |||
 |
info@kemiworks.net sales@kemiworks.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Riverland Trading LLC. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (336).944-2293 | |||
 |
bens@riverlandtrading.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2023 | ||||
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Strem Chemicals, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (978) 499-1600 | |||
 |
info@strem.com | |||
| Chemical manufacturer | ||||
| Classification | Organic raw materials >> Inorganic acid ester |
|---|---|
| Name | Triethyl phosphite |
| Synonyms | Phosphorous acid triethyl ester |
| Molecular Structure | 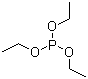 |
| Molecular Formula | C6H15O3P |
| Molecular Weight | 166.16 |
| CAS Registry Number | 122-52-1 |
| EC Number | 204-552-5 |
| SMILES | CCOP(OCC)OCC |
| Density | 0.969 g/mL (Expl.) |
|---|---|
| Melting point | -112 ºC (Expl.) |
| Boiling point | 159.8±8.0 ºC 760 mmHg (Calc.)*, 156 ºC (Expl.) |
| Flash point | 54.1±18.7 ºC (Calc.)*, 54.4 ºC (Expl.) |
| Solubility | water: slightly soluble (Expl.) |
| Refraction index | 1.413 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |

 GHS02;GHS07 WarningGHS02 Details
GHS02;GHS07 WarningGHS02 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H226-H302-H315-H317-H319-H332-H335-H412 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P233-P240-P241-P242-P243-P261-P264-P264+P265-P270-P271-P272-P273-P280-P301+P317-P302+P352-P303+P361+P353-P304+P340-P305+P351+P338-P317-P319-P321-P330-P332+P317-P333+P317-P337+P317-P362+P364-P370+P378-P403+P233-P403+P235-P405-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 2323 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Triethyl phosphite is an organophosphorus compound with the molecular formula C6H15PO3. Structurally, it consists of a phosphorus atom bonded to three ethoxy groups (–OCH2CH3), forming a trialkyl phosphite ester. It is a colorless, flammable liquid with a mild, characteristic odor and is soluble in most organic solvents while having limited solubility in water. Triethyl phosphite is widely used as a reagent in organic synthesis. Its nucleophilic phosphorus center allows it to react with alkyl halides, acyl halides, and other electrophiles. One of its primary applications is in the **Michaelis–Arbuzov reaction**, in which triethyl phosphite reacts with alkyl halides to produce diethyl phosphonates. These phosphonates are important intermediates in the synthesis of pharmaceuticals, agrochemicals, and other organophosphorus compounds. Beyond the Michaelis–Arbuzov reaction, triethyl phosphite can be used in the preparation of phosphinates, phosphonates, and related derivatives through nucleophilic substitution or addition reactions. Its liquid form at room temperature, relatively low toxicity, and stability under standard laboratory conditions make it convenient for handling in both academic and industrial settings. Triethyl phosphite is typically synthesized by reacting phosphorus trichloride with ethanol, substituting the chlorine atoms with ethoxy groups. The reaction requires careful control of temperature and moisture to avoid side reactions and to ensure high-purity product. Standard safety precautions must be observed, as the compound is flammable and reacts with strong oxidizing agents. Overall, triethyl phosphite is a versatile organophosphorus reagent, notable for its nucleophilic reactivity and utility in forming phosphorus-containing intermediates. Its role in the Michaelis–Arbuzov reaction and other synthetic transformations makes it an essential reagent in organic and organophosphorus chemistry. References 2018. Rhenium and technetium complexes of thioamide derivatives of pyridylhydrazine that bind to amyloid-β plaques. Journal of Biological Inorganic Chemistry, 23(6). DOI: 10.1007/s00775-018-1590-4 2017. Synthesis of Lactones and Other Heterocycles. Topics in Current Chemistry, 375(2). DOI: 10.1007/s41061-017-0108-9 |
| Market Analysis Reports |
| List of Reports Available for Triethyl phosphite |