Online Database of Chemicals from Around the World
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Amine |
|---|---|
| Name | 4-(Hexadecyloxy)benzene-1,3-diamine |
| Molecular Structure | 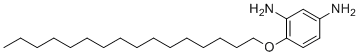 |
| Molecular Formula | C22H40N2O |
| Molecular Weight | 348.57 |
| CAS Registry Number | 137819-03-5 |
| SMILES | CCCCCCCCCCCCCCCCOC1=C(C=C(C=C1)N)N |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P280-P301+P312-P302+P352-P305+P351+P338 Details |
| SDS | Available |
|
4-(Hexadecyloxy)benzene-1,3-diamine is an aromatic diamine bearing a long linear alkoxy substituent, and it belongs to a class of functional organic compounds that combine rigid aromatic cores with flexible aliphatic chains. Such molecular architectures have attracted sustained attention in organic synthesis and materials chemistry because they integrate chemical reactivity associated with aromatic amines with physicochemical properties imparted by hydrophobic alkyl chains. The discovery and study of alkoxy-substituted aromatic diamines emerged from broader investigations into substituted anilines and phenylenediamines during the twentieth century. Aromatic diamines were initially explored as intermediates in dye chemistry, polymer science, and pharmaceuticals. Introduction of long-chain alkoxy groups, such as hexadecyloxy substituents, was later recognized as an effective strategy to tune solubility, melting behavior, and self-assembly characteristics. This structural modification allows otherwise rigid aromatic amines to exhibit amphiphilic behavior, which is valuable in applications requiring compatibility with nonpolar media or organized molecular assemblies. The synthesis of 4-(hexadecyloxy)benzene-1,3-diamine typically relies on classical aromatic substitution strategies combined with ether formation reactions. A common approach involves the preparation of a suitably substituted nitro or amino phenol, followed by O-alkylation with a long-chain alkyl halide such as 1-bromohexadecane under basic conditions to install the hexadecyloxy group. Subsequent reduction steps convert nitro groups to amino functionalities when required. These methods are well established in the literature for related alkoxy-substituted anilines and diamines and are favored for their reliability and scalability. Careful control of reaction conditions is essential to avoid over-alkylation or degradation of the aromatic amine groups. In terms of applications, 4-(hexadecyloxy)benzene-1,3-diamine is primarily valued as a building block rather than as an end-use chemical. Its diamine functionality enables participation in condensation reactions with diacids, diisocyanates, or electrophilic aromatic substitution partners, making it useful in the synthesis of functional polymers and oligomers. The presence of the long alkyl chain can impart flexibility, hydrophobicity, and processability to polymeric materials derived from this compound. Such features are particularly attractive in specialty polyamides, polyimides, and polyurethane systems designed for coatings, adhesives, or advanced composites. Another important area of application lies in materials designed for molecular self-organization. Alkoxy-substituted aromatic diamines are known to exhibit liquid-crystalline or semi-ordered behavior when incorporated into larger molecular frameworks. The hexadecyl chain promotes van der Waals interactions and phase separation from polar domains, while the aromatic diamine core supports directional interactions such as hydrogen bonding. As a result, derivatives of 4-(hexadecyloxy)benzene-1,3-diamine have been investigated as precursors for functional soft materials, including organic electronic components, sensors, and surface-modifying agents. The compound is also of interest in dye and pigment chemistry. Aromatic diamines are classic intermediates in azo dye synthesis, and substitution with long alkyl chains can improve dye affinity for hydrophobic substrates or alter aggregation behavior in formulations. Although 4-(hexadecyloxy)benzene-1,3-diamine itself is not typically used directly as a dye, its structural motif is representative of intermediates employed to tailor colorant properties for plastics, coatings, and inks. Overall, 4-(hexadecyloxy)benzene-1,3-diamine exemplifies how combining traditional aromatic amine chemistry with long-chain alkyl substitution expands the functional scope of organic compounds. Its discovery is rooted in established aromatic substitution chemistry, while its applications reflect modern demands in materials science for molecules that bridge rigidity and flexibility, polarity and hydrophobicity. References Baranov NI, Bagrii EI, Safir RE, Cherednichenko AG, Bozhenko KV, Maksimov AL (2024) Quantum-chemical study of formation of alkyl- and alkenyladamantanes by ionic alkylation with olefins. Kinetics and Catalysis 65(4) 417–428 DOI: 10.1134/S0023158423601171 ChenChen Fang, YongQiang Xiong, Yun Li, QianYong Liang, TongShan Wang, YongXin Li (2015) The effect of volatile components in oil on evolutionary characteristics of diamondoids during oil thermal pyrolysis. Science China Earth Sciences 58(10) 1785–1796 DOI: 10.1007/s11430-015-5163-x Ivanova AE, Kanat’eva AY, Kurganov AA, Selifanova MV, Purygin PP (2017) Aerobic degradation of adamantanes at highly acidic conditions. Microbiology 86(3) 346–353 DOI: 10.1134/S0026261717030067 |
| Market Analysis Reports |
| List of Reports Available for 4-(Hexadecyloxy)benzene-1,3-diamine |