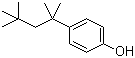
4-tert-Octylphenol is an alkylphenol compound with the molecular formula C14H22O. Structurally, it consists of a phenol ring substituted at the para position with a tert-octyl group, a branched eight-carbon alkyl chain. This combination of a hydrophobic alkyl chain and a polar hydroxyl group imparts unique physical and chemical properties. The compound is typically a viscous, colorless to pale yellow liquid at room temperature, with low solubility in water but good solubility in organic solvents such as ethanol, acetone, and hydrocarbons.
4-tert-Octylphenol is primarily used as a precursor in the production of industrial chemicals. It serves as a key raw material in the synthesis of nonionic surfactants, alkylphenol ethoxylates, and other detergent components. Its phenolic hydroxyl group can undergo alkylation, sulfonation, or ethoxylation, enabling the formation of a wide range of derivatives used in cleaning agents, emulsifiers, and wetting agents.
The compound is also employed in the production of antioxidants, plastic stabilizers, and epoxy resin curing agents. The sterically hindered tert-octyl group provides stability to phenolic derivatives by reducing susceptibility to oxidation, which is valuable in polymer and material applications where thermal and oxidative resistance is important.
In addition to industrial applications, 4-tert-octylphenol has been studied in environmental chemistry due to its persistence and potential as an endocrine-disrupting compound. Its hydrophobic nature leads to accumulation in sediments and biota, raising concerns about environmental impact and prompting monitoring in water and wastewater systems.
Synthetically, 4-tert-octylphenol is typically produced via the acid-catalyzed alkylation of phenol with tert-octyl alcohol or isobutylene-derived oligomers. The reaction yields the para-substituted product predominantly due to steric effects, with minor amounts of ortho-substituted isomers. Purification usually involves distillation or crystallization to achieve the desired product purity.
Overall, 4-tert-octylphenol is a versatile alkylphenol used extensively as a chemical intermediate in surfactants, polymer stabilizers, and other industrial products. Its combination of a phenolic hydroxyl group and a bulky hydrophobic alkyl chain provides both reactivity and stability, making it a valuable building block in chemical manufacturing.
References
2025. Exposure risk assessment through toxicodynamics assessment of 4-tert-octylphenol and estimation of new reference values based on human tissue toxicity. Environmental Pollution.
DOI: 10.1016/j.envpol.2025.125969
2025. The resveratrol attenuates reactive oxygen species mediated DNA damage in cardiac malformations caused by 4-tert-octylphenol. Toxicology and Applied Pharmacology.
DOI: 10.1016/j.taap.2025.117284
|




















 GHS05;GHS09 Danger Details
GHS05;GHS09 Danger Details