Online Database of Chemicals from Around the World
| MolScanner | Singapore | Inquire | ||
|---|---|---|---|---|
 |
+86 18621675448 | |||
 |
marketing@molscanner.com | |||
 |
WhatsApp: 9896 7603 | |||
| Chemical manufacturer since 2025 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Heterocyclic compound >> Piperidines |
|---|---|
| Name | (2S)-tert-Butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate |
| Synonyms | tert-butyl (2S)-3-cyano-2-methyl-4-oxopiperidine-1-carboxylate |
| Molecular Structure | 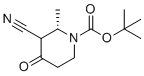 |
| Molecular Formula | C12H18N2O3 |
| Molecular Weight | 238.28 |
| CAS Registry Number | 2212021-56-0 |
| SMILES | C[C@H]1C(C(=O)CCN1C(=O)OC(C)(C)C)C#N |
| Density | 1.1±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 379.3±42.0 ºC 760 mmHg (Calc.)* |
| Flash point | 183.2±27.9 ºC (Calc.)* |
| Index of refraction | 1.495 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details |
|---|---|
| Hazard Statements | H302-H315-H319-H335 Details |
| Precautionary Statements | P261-P264-P270-P271-P280-P301+P312-P302+P352-P304+P340-P305+P351+P338-P330-P332+P313-P337+P313-P362-P403+P233-P405-P501 Details |
| SDS | Available |
|
(2S)-tert-Butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate is a compound with a complex molecular structure that has garnered attention due to its potential applications in synthetic chemistry and medicinal chemistry. This molecule contains a piperidine ring, a cyano group, a carbonyl group, and a tert-butyl ester, all of which contribute to its chemical reactivity and potential utility in various synthetic processes. The discovery of (2S)-tert-butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate is likely tied to advances in the development of chiral piperidine derivatives, which are important in the synthesis of biologically active compounds. The stereochemistry of this molecule, with its (2S) configuration, means it has a specific three-dimensional arrangement that can influence how it interacts with other molecules, particularly biological targets. The (2S) configuration is significant in many drug molecules, as it often corresponds to the form that is most active in biological systems, thereby making compounds with this configuration of particular interest in medicinal chemistry. The piperidine ring is a common structural feature found in many natural products and pharmaceuticals, especially in alkaloids. Its versatility as a building block in drug discovery stems from its ability to engage in various types of chemical reactions, including nucleophilic substitution, reduction, and functional group interconversion. The presence of the piperidine ring in (2S)-tert-butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate suggests that it could be used as a scaffold for designing new drugs targeting specific biological receptors or enzymes. The cyano group, located at position 3 of the piperidine ring, is another key feature of this compound. Cyano groups are known to be electron-withdrawing, which can influence the reactivity of the molecule and its interactions with other substances. This property makes cyano-containing compounds valuable in a variety of chemical reactions, including nucleophilic substitutions and additions. Additionally, the cyano group can be converted into other functional groups, such as amines or carboxylic acids, which increases the potential synthetic applications of the compound. The carbonyl group at position 4, forming an oxo group, adds another level of reactivity to the molecule. Carbonyl-containing compounds are widely used in organic synthesis due to their ability to undergo various reactions, such as nucleophilic additions and reductions. This functionality may be exploited in the synthesis of more complex molecules, particularly those involving amide or ester linkages, which are common in drug molecules and biologically active compounds. The tert-butyl ester group at position 1 of the piperidine ring is a bulky protecting group that can provide steric hindrance and prevent unwanted reactions at this site. Ester groups are commonly used in organic synthesis to protect alcohols or carboxylic acids from undesired reactivity during complex synthetic procedures. The steric bulk of the tert-butyl group can also influence the solubility and permeability of the molecule, which may affect its ability to interact with biological systems. (2S)-tert-butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate can be synthesized using standard organic reactions. The piperidine ring can be introduced through cyclization reactions, while the cyano group and ester group can be added via nucleophilic substitution and esterification reactions, respectively. The specific stereochemistry at position 2 of the piperidine ring may be controlled through the use of chiral reagents or catalysts in the synthetic process. The potential applications of (2S)-tert-butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate lie primarily in its use as a building block for more complex molecules. Due to its functional groups, including the cyano, carbonyl, and ester moieties, the compound could serve as an intermediate in the synthesis of pharmaceuticals, agrochemicals, or other biologically active compounds. The piperidine structure is known for its role in the development of drugs that target central nervous system receptors, enzymes, and other biological pathways. Therefore, this compound may be explored for its potential therapeutic properties, particularly in the context of drug discovery. In conclusion, (2S)-tert-butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate is a compound with significant potential in organic synthesis and medicinal chemistry. Its functional groups make it a versatile intermediate for the construction of more complex molecules, and its stereochemistry suggests that it may have useful properties in biological applications. As such, this compound could play a role in the development of new drugs or other bioactive molecules in the future. |
| Market Analysis Reports |
| List of Reports Available for (2S)-tert-Butyl 3-cyano-2-methyl-4-oxopiperidine-1-carboxylate |