Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Zhejiang Huangyan Zhongxing Flavors & Fragrances Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (576) 8417-9161 | |||
 |
trade@zxff.com | |||
| Chemical manufacturer since 1993 | ||||
| chemBlink standard supplier since 2007 | ||||
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Leping Zhongsheng Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (798) 670-2188 | |||
 |
sales@zhongshengchem.com | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2008 | ||||
| Ring Specialty Chemicals Inc. | Canada | Inquire | ||
|---|---|---|---|---|
 |
+1 (416) 493-6870 | |||
 |
info@ringchemicals.com | |||
| Chemical distributor | ||||
| chemBlink standard supplier since 2010 | ||||
| Jiangxi Shengwei Aromatic Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (794) 525-9909 | |||
 |
hl.tz@163.com | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2011 | ||||
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Epsilon Chimie Chemical Manufacturer | France | Inquire | ||
|---|---|---|---|---|
 |
+33 (2) 9842-4650 | |||
 |
pierre.cornec@epsilon-chimie.com | |||
| Chemical manufacturer | ||||
| Classification | Chemical reagent >> Organic reagent >> Carboxylic anhydride |
|---|---|
| Name | Ethyl dimethylphosphonoacetate |
| Synonyms | Ethoxycarbonylmethylphosphonic acid dimethyl ester; Phosphonoacetic acid P,P-dimethyl ethyl ester |
| Molecular Structure | 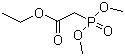 |
| Molecular Formula | C6H13O5P |
| Molecular Weight | 196.14 |
| CAS Registry Number | 311-46-6 |
| EC Number | 206-222-6 |
| SMILES | CCOC(=O)CP(=O)(OC)OC |
| Density | 1.2±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 268.7 ºC 760 mmHg (Calc.)* |
| Flash point | 98.9 ºC (Calc.)* |
| Index of refraction | 1.414 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319 Details | ||||||||||||||||
| Precautionary Statements | P264-P264+P265-P280-P302+P352-P305+P351+P338-P321-P332+P317-P337+P317-P362+P364 Details | ||||||||||||||||
| Hazard Classification | |||||||||||||||||
| |||||||||||||||||
| SDS | Available | ||||||||||||||||
|
Ethyl dimethylphosphonoacetate is an organophosphorus compound with the molecular formula C5H11O4P. Structurally, it consists of a phosphonate group bearing two methyl substituents connected via a methylene bridge to an ethyl ester of acetic acid. This combination of a stabilized phosphonate group and an ester functionality imparts distinctive reactivity, making it a useful reagent in carbon–carbon bond-forming reactions. The compound is typically a colorless to pale yellow liquid, soluble in organic solvents and sparingly soluble in water. The primary application of ethyl dimethylphosphonoacetate is in the Horner–Wadsworth–Emmons reaction, a variant of the Wittig olefination. In this reaction, the methylene group adjacent to the phosphonate is deprotonated to form a stabilized carbanion, which then reacts with aldehydes or ketones to generate α,β-unsaturated esters. The phosphonate group stabilizes the anion, allowing reactions under milder conditions and often providing high stereochemical selectivity. Ethyl dimethylphosphonoacetate is also used as a building block in the synthesis of heterocycles, pharmaceuticals, and agrochemicals. The ester functionality allows for subsequent transformations, such as hydrolysis to a carboxylic acid or reduction to an alcohol, providing versatility in multistep syntheses. Synthetically, the compound can be prepared by the reaction of diethyl methylphosphonate with ethyl bromoacetate or through other alkylation strategies involving phosphonate esters. Handling requires standard laboratory precautions, as it is flammable and reacts with strong bases used to generate the reactive carbanion. Overall, ethyl dimethylphosphonoacetate is a versatile organophosphorus reagent valued for its nucleophilic reactivity and stability. Its role in the Horner–Wadsworth–Emmons reaction and related transformations makes it a key reagent in the synthesis of unsaturated esters and other carbon–carbon bonded frameworks in organic chemistry. References 2017. Asymmetric Synthetic Strategies of (R)-(-)-Baclofen: An Antispastic Drug. Synthesis, 49(20). DOI: 10.1055/s-0036-1590938 |
| Market Analysis Reports |
| List of Reports Available for Ethyl dimethylphosphonoacetate |