Online Database of Chemicals from Around the World
| Shanghai Worldyang Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13651600618 +86 (21) 5679-5779 | |||
 |
sales7777@worldyachem.com | |||
 |
QQ chat | |||
 |
WeChat: 13651600618 | |||
 |
WhatsApp: +86 13651600618 | |||
| Chemical manufacturer since 2012 | ||||
| chemBlink premium supplier since 2023 | ||||
| Hangzhou Verychem Science And Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8816-2785 +86 13606544505 | |||
 |
lucy@verychem.com | |||
| Chemical manufacturer since 2004 | ||||
| chemBlink massive supplier since 2021 | ||||
| Classification | Organic raw materials >> Aldehyde |
|---|---|
| Name | 2,5-Furandicarboxylic acid |
| Synonyms | Furan-2,5-dicarboxylic acid |
| Molecular Structure | 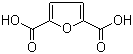 |
| Molecular Formula | C6H4O5 |
| Molecular Weight | 156.09 |
| CAS Registry Number | 3238-40-2 |
| EC Number | 221-800-8 |
| SMILES | C1=C(OC(=C1)C(=O)O)C(=O)O |
| Density | 1.6±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 320 ºC (Expl.) |
| Boiling point | 419.2±30.0 ºC 760 mmHg (Calc.)* |
| Flash point | 207.3±24.6 ºC (Calc.)* |
| Index of refraction | 1.581 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
2,5-Furandicarboxylic acid is a bio-based aromatic diacid that has gained prominence as a renewable alternative to petroleum-derived monomers. Structurally, it contains a furan ring substituted with two carboxylic acid groups at the 2- and 5-positions, a symmetry that enables polymer formation similar to the well-known terephthalic acid. Its importance has been amplified by global interest in sustainable materials, since it can be produced from biomass-derived carbohydrates rather than fossil feedstocks. The first isolation of 2,5-furandicarboxylic acid was reported in the late nineteenth century through oxidative degradation of naturally occurring furan derivatives. Modern production relies on catalytic oxidation of 5-hydroxymethylfurfural, a key platform molecule obtained from dehydration of fructose or cellulosic sugars. Catalysts such as supported noble metals and metal oxides have been used to optimize yields, selectivity, and reaction conditions. Because the synthetic pathway begins with sugars, the compound occupies an important position in the broader effort to transform agricultural and forestry waste into industrial chemicals. Interest in this molecule increased significantly when it was identified as a potential component for high-performance polyesters. The combination of aromaticity and the furan ring’s electron-rich character gives its derivatives mechanical and thermal properties comparable to or in some cases exceeding those of traditional petrochemical plastics. Polyethylene furanoate, synthesized from 2,5-furandicarboxylic acid and ethylene glycol, has attracted attention as a renewable substitute for polyethylene terephthalate. Its barrier properties toward gases such as carbon dioxide and oxygen are often superior, enabling potential use in beverage bottles, food packaging, and other applications requiring controlled permeability. The compound has also been applied in the synthesis of polyamides, polyurethanes, and specialty copolymers. Its bifunctional carboxyl groups enable straightforward polycondensation reactions. Work on derivatives such as esters, anhydrides, and activated intermediates has broadened the range of polymer architectures accessible from this bio-based monomer. Because the furan ring can engage in electronic interactions that differ from those of benzene-based monomers, materials incorporating 2,5-furandicarboxylic acid often show distinct optical and mechanical behavior. Another area of application is in plasticizer and solvent design. Esterification of the two carboxyl groups generates a family of dialkyl furandicarboxylates that have been explored for use as more sustainable alternatives to conventional plasticizers. Their thermal stability and low volatility align with growing regulatory and consumer interest in safer, non-phthalate plasticizer systems. Some derivatives have also been evaluated as renewable solvents for industrial processes. Beyond polymer science, 2,5-furandicarboxylic acid has been investigated for electrochemical applications. Because its reduction produces a stable and reversible redox couple, salts and derivatives of the molecule have been evaluated for potential use in organic electrode materials. Research in this direction continues, driven by interest in metal-free and low-cost battery chemistries. From a materials development perspective, the compound benefits from strong global research momentum. Pilot-scale manufacturing has demonstrated pathways to scale up production from carbohydrate feedstocks, an essential step toward commercial viability. Efforts to improve catalytic oxidation, minimize by-product formation, and integrate biomass conversion with downstream polymerization continue to shape its industrial future. Its position as a representative bio-aromatic monomer makes it a cornerstone of emerging renewable polymer platforms. References Rosatella, A. A., Simeonov, S. P., Frade, R. F. M. & Afonso, C. A. M. (2011). 5-Hydroxymethylfurfural as a building block platform for bio-based chemicals and materials. Green Chemistry, 13, 754–793. [https://doi.org/10.1039/C0GC00401D](https://doi.org/10.1039/C0GC00401D) Werpy, T. & Petersen, G. (2004). Top Value Added Chemicals from Biomass, Volume I: Results of Screening for Potential Candidates from Sugars and Synthesis Gas. U.S. Department of Energy. [https://doi.org/10.2172/15008859](https://doi.org/10.2172/15008859) |
| Market Analysis Reports |
| List of Reports Available for 2,5-Furandicarboxylic acid |