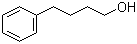
4-Phenylbutanol is an aromatic alcohol consisting of a benzene ring linked through a four-carbon aliphatic chain to a terminal hydroxyl group. This structure places the compound at the intersection of aromatic and aliphatic chemistry, giving it physical and chemical properties that have made it useful as an intermediate in organic synthesis and in applied chemical research. The compound is typically a colorless to pale yellow liquid with moderate viscosity and a faint aromatic odor, and it exhibits limited solubility in water but good solubility in most common organic solvents.
The identification and preparation of 4-phenylbutanol emerged from early studies on phenylalkyl alcohols, which were investigated in the late nineteenth and early twentieth centuries as chemists explored the influence of alkyl chain length on the reactivity and physical behavior of aromatic compounds. Methods for producing phenyl-substituted alcohols developed alongside advances in Friedel–Crafts chemistry, catalytic hydrogenation, and carbonyl reduction reactions. 4-Phenylbutanol was prepared as part of systematic efforts to synthesize and characterize homologous series of phenylalkanols, allowing chemists to correlate boiling points, solubility, and reactivity with molecular structure.
Several established synthetic routes to 4-phenylbutanol have been reported in the chemical literature. One common approach involves the reduction of 4-phenylbutanal using metal hydrides or catalytic hydrogenation. Another well-established method is the hydrogenation of cinnamyl alcohol or related unsaturated precursors, in which the carbon–carbon double bond is reduced while preserving the aromatic ring and hydroxyl functionality. Grignard reactions between phenylmagnesium halides and suitable epoxides or aldehydes have also been used to construct the carbon skeleton, followed by hydrolysis to yield the target alcohol. These routes reflect standard, well-understood transformations in organic chemistry and have been applied reproducibly in laboratory and industrial contexts.
In terms of applications, 4-phenylbutanol is primarily valued as an intermediate rather than as an end-use product. In fragrance and flavor chemistry, phenylalkyl alcohols with varying chain lengths are known to contribute floral or balsamic notes, and 4-phenylbutanol has been examined as a component or precursor in the formulation of aromatic compositions. Its relatively low volatility compared with shorter phenylalkanols allows it to function as a fixative or modifier in such mixtures, influencing persistence and odor profile.
In pharmaceutical and fine chemical synthesis, 4-phenylbutanol serves as a versatile building block. The terminal hydroxyl group can be converted into halides, esters, or ethers, enabling further functionalization, while the phenyl ring provides a stable aromatic framework for substitution or coupling reactions. As a result, the compound has been used in the preparation of more complex molecules, including biologically active substances and advanced intermediates for medicinal chemistry research. Its predictable reactivity makes it useful for exploring structure–activity relationships in series of phenylalkyl derivatives.
From a physical chemistry perspective, 4-phenylbutanol has been employed in studies examining hydrogen bonding, solvation, and the effect of aromatic substitution on alcohol behavior. Its balance of hydrophobic and hydrophilic features makes it suitable for probing interactions in mixed solvent systems and for comparing the properties of aromatic versus purely aliphatic alcohols. Such studies have contributed to a broader understanding of how molecular structure governs intermolecular forces and phase behavior.
Overall, 4-phenylbutanol represents a well-characterized aromatic alcohol whose discovery arose from foundational research into phenyl-substituted organic compounds. Its continued use as a synthetic intermediate, a subject of physical and applied chemical studies, and a component in fragrance-related research underscores its practical relevance. The compound exemplifies how relatively simple organic molecules can maintain long-term importance through their adaptability and reliable chemistry.
References
Weissermel K, Arpe H-J (2008) Industrial Organic Chemistry, 4th edn. Wiley-VCH, Weinheim DOI: 10.1002/9783527619191
|



















 GHS07 Warning Details
GHS07 Warning Details