Online Database of Chemicals from Around the World
| Hubei Innerse Biotechnology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (027) 8818-9299 | |||
 |
sales@innersebio.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2020 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Ketone compound |
|---|---|
| Name | 10-Nonadecanone |
| Synonyms | nonadecan-10-one |
| Molecular Structure | 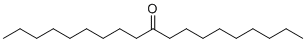 |
| Molecular Formula | C19H38O |
| Molecular Weight | 282.50 |
| CAS Registry Number | 504-57-4 |
| EC Number | 207-994-7 |
| SMILES | CCCCCCCCCC(=O)CCCCCCCCC |
| Density | 0.8±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 58 ºC (Expl.) |
| Boiling point | 351.2±10.0 ºC 760 mmHg (Calc.)*, 412.7 ºC (Expl.) |
| Flash point | 81.6±14.6 ºC (Calc.)* |
| Index of refraction | 1.444 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315 Details | ||||||||||||||||||||||||
| Precautionary Statements | P264-P280-P302+P352-P321-P332+P317-P362+P364 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
10-Nonadecanone is a long-chain aliphatic ketone with the molecular formula C19H38O. Structurally, it consists of a linear 19-carbon hydrocarbon chain with a carbonyl group located at the tenth carbon, placing the ketone roughly in the center of the molecule. This mid-chain position of the carbonyl group influences the physical properties of the compound, giving it characteristics typical of long-chain ketones: it is solid or waxy at room temperature, relatively hydrophobic, and soluble in nonpolar organic solvents but poorly soluble in water. Compounds such as 10-Nonadecanone are commonly found as minor constituents of plant waxes, insect cuticular lipids, and other biological lipid mixtures. In plants, mid-chain ketones contribute to the composition of epicuticular waxes, which serve protective roles including reducing water loss, shielding against ultraviolet radiation, and providing a barrier to pathogens. In insects, similar long-chain ketones can influence desiccation resistance and may function in chemical communication, although the specific role of 10-Nonadecanone in these systems is less well documented than for shorter or more common ketones. From a synthetic perspective, 10-Nonadecanone can be prepared in the laboratory via oxidation of the corresponding secondary alcohol or by constructing the carbon chain through controlled carbon–carbon bond-forming reactions followed by introduction of the ketone functionality. Its structure and properties make it a useful reference compound in analytical chemistry, particularly for gas chromatography and mass spectrometry, where it serves as a standard for identifying mid-chain aliphatic ketones in natural waxes and lipid extracts. Applications of 10-Nonadecanone are mainly research-oriented. It is used to study the physical and chemical properties of long-chain ketones, their behavior in biological systems, and their role in surface chemistry. Additionally, it can serve as a model compound in the exploration of bio-based chemicals derived from fatty acids, where such ketones may be intermediates in the production of waxes, surfactants, or other hydrophobic materials. Overall, 10-Nonadecanone is a long-chain mid-ketone whose significance lies in its presence in natural waxes, its utility as a reference standard, and its role as a model compound in research on long-chain lipids and bio-based chemical materials. Its combination of a central carbonyl group and a long hydrocarbon chain exemplifies the properties of mid-chain aliphatic ketones, influencing melting point, solubility, and reactivity in both natural and synthetic contexts. References 2023. The LOTUS Initiative for Open Natural Products Research: frozen dataset union wikidata (with metadata). Zenodo. DOI: 10.5281/zenodo.5794106 2021. Multitudinous Potential of Trichoderma Species in Imparting Resistance Against F. oxysporum f. sp. cucumerinum and Meloidogyne incognita Disease Complex. Journal of Plant Growth Regulation. DOI: 10.1007/s00344-021-10372-9 2012. Volatile Mediated Interactions Between Bacteria and Fungi in the Soil. Journal of Chemical Ecology, 38(6). DOI: 10.1007/s10886-012-0135-5 |
| Market Analysis Reports |
| List of Reports Available for 10-Nonadecanone |