Online Database of Chemicals from Around the World
| Capot Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8558-6718 +86 13336195806 | |||
 |
capotchem@gmail.com sales@capotchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2006 | ||||
| Adam, Gates & Company, LLC | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (908) 237-0300 | |||
 |
sales@adamgatescompany.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Luminochem Ltd. | Hungary | Inquire | ||
|---|---|---|---|---|
 |
+36 (20) 239-9401 | |||
 |
info@luminochem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2012 | ||||
| Finetech Industry Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (27) 8746-5837 +86 18971612321 | |||
 |
sales@finetechnology-ind.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2009 | ||||
| chemBlink standard supplier since 2019 | ||||
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Organica Feinchemie GmbH | Germany | Inquire | ||
|---|---|---|---|---|
 |
+49 (3494) 63-6215 | |||
 |
dmi@weylchem-organica.com | |||
| Chemical manufacturer | ||||
| Classification | Pharmaceutical intermediate >> Heterocyclic compound intermediate >> Pyrimidine compound >> Amine |
|---|---|
| Name | Tris(4-aminophenyl)amine |
| Synonyms | N,N-Bis(4-aminophenyl)benzene-1,4-diamine |
| Molecular Structure | 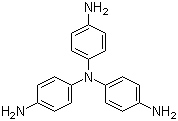 |
| Molecular Formula | C18H18N4 |
| Molecular Weight | 290.36 |
| CAS Registry Number | 5981-09-9 |
| EC Number | 227-791-7 |
| SMILES | C1=CC(=CC=C1N)N(C2=CC=C(C=C2)N)C3=CC=C(C=C3)N |
| Density | 1.3±0.1 g/cm3 Calc.* |
|---|---|
| Melting point | 230 ºC (Expl.) |
| Boiling point | 571.3±45.0 ºC 760 mmHg (Calc.)* |
| Flash point | 294.1±23.5 ºC (Calc.)* |
| Index of refraction | 1.752 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||
| |||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||
|
Tris(4-aminophenyl)amine is an aromatic amine with the molecular formula C18H15N4. Structurally, it consists of a central nitrogen atom bonded to three para-substituted aminophenyl groups. This arrangement results in a tripodal structure with three reactive amino groups extending from a single central nitrogen, combining high nucleophilicity with rigidity from the aromatic system. The compound is typically a solid at room temperature, appearing pale yellow to off-white, and is soluble in polar organic solvents such as dimethylformamide, dimethyl sulfoxide, and ethanol, but sparingly soluble in water. Tris(4-aminophenyl)amine is primarily used as a monomer in polymer chemistry. Its amino groups readily undergo condensation reactions with dianhydrides, diacid chlorides, or epoxides to form polyimides, polyamides, or epoxy networks. The tripodal structure allows for the formation of highly crosslinked polymers with enhanced thermal stability, mechanical strength, and chemical resistance. This makes it especially valuable in high-performance materials for coatings, adhesives, and electronic applications. In addition to polymerization, the amino groups can be functionalized through acylation, sulfonation, or Schiff base formation, expanding its utility in organic synthesis and materials design. Its rigid aromatic framework combined with multiple reactive sites allows the preparation of advanced materials with tailored mechanical, optical, and chemical properties. Synthetically, tris(4-aminophenyl)amine can be prepared via nucleophilic aromatic substitution or Buchwald–Hartwig amination of trihalogenated amine precursors, followed by reduction of any nitro groups to amines. Purification typically involves recrystallization or chromatographic techniques to achieve the desired purity for polymerization or functionalization. Overall, tris(4-aminophenyl)amine is a versatile aromatic amine with three highly reactive amino groups arranged in a tripodal geometry. Its combination of nucleophilic functionality, rigid aromatic structure, and ability to form highly crosslinked networks makes it a valuable intermediate in high-performance polymer synthesis, functional materials, and advanced chemical applications. References 2025. Hydrophobic covalent organic frameworks for solid-phase microextraction of pyrethroids. Microchimica Acta. DOI: 10.1007/s00604-025-07360-1 2025. The influence of building block length on the electrochemical and electrochromic properties of triphenylamine based covalent organic framework. Journal of Materials Science. DOI: 10.1007/s10853-025-10998-0 2025. Structural and functional optimization of COF-coated stirring bars for environmental monitoring and biological sample analysis. Analytical and Bioanalytical Chemistry. DOI: 10.1007/s00216-025-05875-3 |
| Market Analysis Reports |
| List of Reports Available for Tris(4-aminophenyl)amine |