Online Database of Chemicals from Around the World
| Alfa Chemistry | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (201) 478-8534 | |||
 |
inquiry@alfa-chemistry.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2012 | ||||
| Shanghai CR Corporation Limited | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 5736-6208 +86 13917420128 | |||
 |
fred.wen@crcorporation.cn | |||
 |
QQ chat | |||
| Chemical manufacturer since 2016 | ||||
| chemBlink standard supplier since 2017 | ||||
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Chemical reagent >> Organic reagent >> Alkane |
|---|---|
| Name | Bis(4-aminophenoxy)ethane |
| Synonyms | 1,2-Bis(p-aminophenoxy)ethane |
| Molecular Structure | 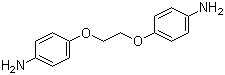 |
| Molecular Formula | C14H16N2O2 |
| Molecular Weight | 244.29 |
| CAS Registry Number | 6052-10-4 |
| EC Number | 828-427-5 |
| SMILES | C1=CC(=CC=C1N)OCCOC2=CC=C(C=C2)N |
| Density | 1.2±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 477.2±30.0 ºC 760 mmHg (Calc.)* |
| Flash point | 273.5±18.2 ºC (Calc.)* |
| Index of refraction | 1.631 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |


 GHS07;GHS08;GHS09 Warning Details
GHS07;GHS08;GHS09 Warning Details | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H302-H315-H319-H335-H341-H411 Details | ||||||||||||||||||||||||
| Precautionary Statements | P203-P261-P264-P264+P265-P270-P271-P273-P280-P301+P317-P302+P352-P304+P340-P305+P351+P338-P318-P319-P321-P330-P332+P317-P337+P317-P362+P364-P391-P403+P233-P405-P501 Details | ||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||
| |||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||
|
Bis(4-aminophenoxy)ethane is an aromatic diamine with the molecular formula C14H16N2O2. Structurally, it consists of an ethane backbone linked at both ends to 4-aminophenoxy groups, in which each phenyl ring bears an amino group at the para position and is connected via an ether linkage to the central ethyl spacer. This combination of nucleophilic amino groups, aromatic rings, and a flexible aliphatic linker imparts both chemical reactivity and solubility suitable for polymer synthesis. The compound typically appears as a pale yellow to off-white solid, soluble in polar organic solvents such as dimethylformamide, acetone, and ethanol, but only sparingly soluble in water. Bis(4-aminophenoxy)ethane is primarily used as a monomer in the synthesis of high-performance polymers. Its amino groups readily undergo condensation with dianhydrides, diacid chlorides, or epoxides to form polyimides, polyamides, and epoxy-based networks. The aromatic rings contribute rigidity and thermal stability, while the ethane ether linker imparts flexibility and improved processability. This combination allows the production of polymers with a desirable balance of mechanical strength, thermal resistance, and chemical stability, suitable for coatings, adhesives, and electronic materials. The amino groups also enable functionalization through reactions such as acylation, sulfonation, or Schiff base formation, expanding the compound’s utility in advanced material synthesis. Its reactivity and structure allow for the design of materials with tailored thermal, mechanical, and surface properties. Synthetically, bis(4-aminophenoxy)ethane is typically prepared by nucleophilic aromatic substitution of 4-nitrophenol with ethylene dibromide or dichloroethane, followed by reduction of the nitro groups to amino groups. Purification is commonly achieved through recrystallization or chromatographic techniques to obtain material suitable for polymerization or further functionalization. Overall, bis(4-aminophenoxy)ethane is a versatile aromatic diamine monomer combining reactive amino groups, a rigid aromatic core, and a flexible ether-linked ethyl spacer. Its structural features make it a valuable intermediate for high-performance polymers, functionalized materials, and other applications requiring a balance of thermal stability, chemical reactivity, and mechanical flexibility. References 2019. Synthesis and optical properties of semi-fluorinated poly(ether imide)s derived from non-fluorinated 1,4-bis(3,4-dicarboxyphenoxy)benzene dianhydride (HQDPA) and trifluoromethyl-substituted diamines. Journal of Materials Research, 34(3). DOI: 10.1557/jmr.2018.440 2010. Novel poly(ether-amide)s derived from 1,4-(4-carboxy phenoxy)butane and ethereal diamines: Synthesis and properties. Macromolecular Research, 18(12). DOI: 10.1007/s13233-010-1213-6 |
| Market Analysis Reports |
| List of Reports Available for Bis(4-aminophenoxy)ethane |