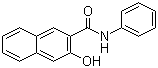
The compound commonly known as Naphthol AS is a key intermediate in the synthesis of classical azo pigments. Its chemical identity is 3-hydroxy-2-naphthanilide, and it belongs to the family of naphtholic coupling components used extensively in pigment and textile-dyeing chemistry. The material emerged in the early twentieth century during rapid industrial expansion of synthetic organic colorants, when naphthol-based coupling systems were developed to improve shade range, fastness, and process reliability in azo pigment formation. Naphthol AS became one of the most important members of this group because its anilide structure provides an effective balance of reactivity and stability, allowing controlled diazo coupling with a wide variety of diazonium salts.
Its discovery is tied to the broader development of naphthol coupling components derived from modified 2-naphthoic and 3-hydroxy-naphthalene frameworks. Manufacturers sought materials that produced bright, durable red and orange azo pigments when reacted in situ on fiber or in pigment formulations. Naphthol AS fulfills this role through its activated aromatic ring, which undergoes electrophilic substitution with diazonium species to form insoluble azo dyes directly within textile fibers. This strategy, known as the naphthol dyeing process, became a major technique in cotton coloration. The insolubility of the final pigment within the fiber confers excellent wash and light fastness, making the system suitable for commercial applications long before more modern reactive dyes were discovered.
Beyond textiles, Naphthol AS also gained importance in the manufacture of high-performance organic pigments. Its derivatives participate in the formation of azo pigments with good dispersibility and strong chromatic properties, which led to their adoption in paints, inks, plastics, and printing systems. In pigment-grade syntheses, Naphthol AS is typically converted to its metal-complexed or laked forms after coupling, improving opacity and stability. In the coatings industry, pigments derived from Naphthol AS became valued for their brightness and versatility, occupying a niche between simpler azo dyes and more costly high-performance pigments.
The compound’s reaction behavior has been investigated extensively, with particular attention to its coupling kinetics, tautomeric balance between amide and enol forms, and the effects of substitution on shade and fastness. These studies helped establish structure–property relationships that guided the design of later coupling components, including halogenated or alkylated analogues that modify hue or reactivity. Naphthol AS also contributed to the understanding of azo dye formation mechanisms, as its relatively robust scaffold allowed systematic study of substituent effects on electrophilic aromatic substitution.
Industrial processes for Naphthol AS typically begin with acylation of aniline derivatives followed by cyclization or rearrangement steps to generate the substituted naphthamide core. These methods have been optimized over decades for yield, purity, and environmental considerations. Although newer dye technologies have reduced reliance on traditional naphthol systems in some sectors, Naphthol AS remains in production due to the unique performance characteristics of its pigments, particularly in applications requiring high fastness at relatively low cost.
In modern practice, Naphthol AS continues to serve as a reference compound in studies on azo coupling, pigment engineering, and structure–reactivity correlations in aromatic amides. Its longstanding industrial history ensures that it remains part of the toolkit for colorant chemistry, bridging classic dyeing technology with contemporary pigment research.
References
Hunger, K. (Ed.). (2003). Industrial Dyes: Chemistry, Properties, Applications. Wiley-VCH. DOI: 10.1002/3527602011
|














 GHS07;GHS09 Warning Details
GHS07;GHS09 Warning Details