Online Database of Chemicals from Around the World
| Simagchem Corporation | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13806087780 | |||
 |
sale@simagchem.com | |||
| Chemical manufacturer since 2002 | ||||
| chemBlink standard supplier since 2008 | ||||
| Jiangsu Kangheng Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (513) 8768-6609 | |||
 |
zkpeasy@hotmail.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2009 | ||||
| Sinocompound Catalysts Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (512) 6721-6630 | |||
 |
sales@sinocompound.com | |||
| Chemical manufacturer since 2008 | ||||
| chemBlink standard supplier since 2010 | ||||
| Hefei TNJ Chemical Industry Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (551) 6541-8684 | |||
 |
sales@tnjchem.com | |||
| Chemical manufacturer since 2001 | ||||
| chemBlink standard supplier since 2010 | ||||
| Shanghai Chang-Gen Chemical Technology Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6910-6824 | |||
 |
sales@shcgchem.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| BOC Sciences | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (631) 485-4226 | |||
 |
info@bocsci.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2010 | ||||
| Ereztech LLC | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (888) 658-1221 | |||
 |
sales@ereztech.com | |||
| Chemical distributor since 2010 | ||||
| chemBlink standard supplier since 2011 | ||||
| Jiangxi Xinxin Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 15957176628 | |||
 |
sales@sinophos.com.cn | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2014 | ||||
| Classification | Organic raw materials >> Organic phosphine compound |
|---|---|
| Name | Tributylphosphane |
| Synonyms | Tris(butyl)phosphine; Tri-n-butylphosphine; TBUP |
| Molecular Structure | 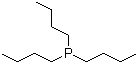 |
| Molecular Formula | C12H27P |
| Molecular Weight | 202.32 |
| CAS Registry Number | 998-40-3 |
| EC Number | 213-651-2 |
| SMILES | CCCCP(CCCC)CCCC |
| Density | 0.82 g/mL (Expl.) |
|---|---|
| Melting point | -65 ºC (Expl.) |
| Boiling point | 244.8 ºC 760 mmHg (Calc.)*, 257.9 ºC (Expl.) |
| Flash point | 112.2±25.0 ºC (Calc.)*, 37.2 ºC (Expl.) |
| Solubility | water: Insoluble (Expl.) |
| Refraction index | 1.462 (Expl.) |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
| Hazard Symbols |



 GHS02;GHS05;GHS07;GHS09 DangerGHS02 Details
GHS02;GHS05;GHS07;GHS09 DangerGHS02 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H226-H250-H251-H302-H312-H314-H318-H411 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Precautionary Statements | P210-P222-P231-P233-P235-P240-P241-P242-P243-P260-P264-P264+P265-P270-P273-P280-P301+P317-P301+P330+P331-P302+P335+P334-P302+P352-P302+P361+P354-P303+P361+P353-P304+P340-P305+P354+P338-P316-P317-P321-P330-P362+P364-P363-P370+P378-P391-P403+P235-P405-P407-P410-P413-P420-P501 Details | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||
| Transport Information | UN 3254 | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Tributylphosphane is an organophosphorus compound with the molecular formula C12H27P. Structurally, it consists of a phosphorus atom bonded to three n-butyl groups, giving a pyramidal geometry with a lone pair of electrons on the phosphorus center. This lone pair makes the compound a strong nucleophile and a good ligand for transition metals. Tributylphosphane is typically a colorless to pale yellow liquid with a characteristic fishy odor, soluble in organic solvents such as hexane, toluene, and dichloromethane, but insoluble in water. The compound is primarily used as a ligand in coordination chemistry and homogeneous catalysis. Its nucleophilic phosphorus center readily coordinates to metals such as palladium, platinum, or rhodium, forming stable complexes that facilitate a variety of catalytic transformations, including hydrogenation, hydroformylation, and cross-coupling reactions. The flexible butyl substituents provide steric bulk that can influence the selectivity and activity of the catalytic complexes. Tributylphosphane is also employed as a reducing agent and nucleophilic reagent in organic synthesis. It can reduce certain organohalides, sulfonyl compounds, and oxidized phosphorus species, and it is frequently used in the synthesis of phosphonium salts for use in Wittig reactions and other phosphorus-mediated transformations. The compound is typically synthesized by the reaction of phosphorus trichloride with n-butyl magnesium halide or n-butyl lithium, followed by careful purification to remove unreacted starting materials and byproducts. Handling requires precautions due to its flammability, toxicity, and strong odor. It is also air-sensitive and may oxidize slowly upon prolonged exposure to oxygen, so it is often stored under inert atmosphere. Overall, tributylphosphane is a versatile organophosphorus compound valued for its nucleophilic phosphorus center, ability to act as a ligand in catalysis, and utility as a reducing and functionalizing reagent in organic synthesis. Its combination of reactivity and steric properties makes it an essential tool in both academic and industrial chemical applications. References 2011. Acute toxicity of nonylphenols and bisphenol A to the embryonic development of the abalone Haliotis diversicolor supertexta. Ecotoxicology, 20(6). DOI: 10.1007/s10646-011-0672-7 |
| Market Analysis Reports |
| List of Reports Available for Tributylphosphane |