Online Database of Chemicals from Around the World
| SL Drugs and Pharmaceuticals Pvt. Ltd. | India | Inquire | ||
|---|---|---|---|---|
 |
+91 (40) 6661-1133 | |||
 |
enquiry@sldrugs.com | |||
| Chemical distributor since 1999 | ||||
| chemBlink standard supplier since 2010 | ||||
| Biosynth AG. | Switzerland | Inquire | ||
|---|---|---|---|---|
 |
+41 (71) 858-2020 | |||
 |
welcome@biosynth.ch | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2014 | ||||
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Achemica | Switzerland | Inquire | ||
|---|---|---|---|---|
 |
+41 (24) 466-2929 | |||
 |
contact@achemica.com | |||
| Chemical manufacturer since 2010 | ||||
| Wuxi Anbao Dyes and Chemicals Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (510) 8580-6868 8586-8628 | |||
 |
james_wang1@yahoo.com | |||
| Chemical manufacturer since 1992 | ||||
| Classification | Organic raw materials >> Amino compound >> Cycloalkylamines, aromatic monoamines, aromatic polyamines and derivatives and salts |
|---|---|
| Name | N-(4-Aminophenyl)-1,4-benzenediamine |
| Synonyms | 4,4'-Iminodianiline |
| Molecular Structure | 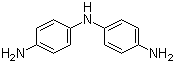 |
| Molecular Formula | C12H13N3 |
| Molecular Weight | 199.25 |
| CAS Registry Number | 537-65-5 |
| EC Number | 208-673-4 |
| SMILES | C1=CC(=CC=C1N)NC2=CC=C(C=C2)N |
| Density | 1.2±0.1 g/cm3 Calc.* |
|---|---|
| Boiling point | 410.7±20.0 ºC 760 mmHg (Calc.)* |
| Flash point | 239.2±25.4 ºC (Calc.)* |
| Index of refraction | 1.733 (Calc.)* |
| * | Calculated using Advanced Chemistry Development (ACD/Labs) Software. |
|
N-(4-Aminophenyl)-1,4-benzenediamine is an aromatic diamine with the molecular formula C12H13N3. Structurally, it consists of a 1,4-diaminobenzene (para-phenylenediamine) core in which one of the amino groups is substituted with a 4-aminophenyl group through a nitrogen–carbon bond. This arrangement results in three amino functionalities distributed across two linked aromatic rings, combining high nucleophilicity with rigidity from the aromatic system. The compound is typically a solid at room temperature, exhibiting pale yellow coloration and good solubility in polar organic solvents such as ethanol, dimethylformamide, and acetone, but limited solubility in water. N-(4-Aminophenyl)-1,4-benzenediamine is primarily used as a building block in polymer synthesis and dye chemistry. Its amino groups can undergo condensation reactions with dianhydrides, diacid chlorides, or aldehydes to form polyimides, polyamides, or Schiff base derivatives. The presence of three amino groups provides multiple sites for crosslinking, allowing the formation of highly functionalized polymers with enhanced mechanical strength, thermal stability, and chemical resistance. In addition, the compound serves as a precursor for the synthesis of azo and heterocyclic dyes. The aromatic amino groups can be diazotized and coupled to other aromatic compounds, forming conjugated chromophores with diverse optical properties. This makes it valuable in the production of colorants, pigments, and functional dyes for industrial applications. Synthetically, N-(4-Aminophenyl)-1,4-benzenediamine can be prepared via selective amination of 1,4-diaminobenzene with 4-nitrochlorobenzene, followed by reduction of the nitro group to the amino group. Careful control of reaction conditions ensures selective substitution and minimizes formation of undesired byproducts. The purified compound is typically obtained through recrystallization or chromatographic techniques. Overall, N-(4-Aminophenyl)-1,4-benzenediamine is a multifunctional aromatic diamine with three reactive amino groups and a rigid bis-phenyl framework. Its combination of nucleophilic sites, structural rigidity, and ability to undergo crosslinking and functionalization makes it a versatile intermediate for high-performance polymers, dyes, and other aromatic amine-based materials. References 2020. Springer Lexikon Kosmetik und K�rperpflege. Springer Lexikon Kosmetik und K�rperpflege. DOI: 10.1007/978-3-662-59127-7_4 2019. Differential pulse voltammetric determination of the carcinogenic diamine 4,4'-oxydianiline by electrochemical preconcentration on a MoS2 based sensor. Microchimica Acta, 186(12). DOI: 10.1007/s00604-019-3906-7 |
| Market Analysis Reports |
| List of Reports Available for N-(4-Aminophenyl)-1,4-benzenediamine |