Online Database of Chemicals from Around the World
| Wilshire Technologies, Inc. | USA | Inquire | ||
|---|---|---|---|---|
 |
+1 (609) 683-1117 | |||
 |
Wilshire-info@evonik.com | |||
| Chemical manufacturer since 1997 | ||||
| chemBlink standard supplier since 2010 | ||||
| Shanghai Hohance Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 3111-5312 | |||
 |
info@hohance.com | |||
| Chemical manufacturer | ||||
| chemBlink standard supplier since 2011 | ||||
| Hangzhou Leap Chem Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8771-1850 | |||
 |
market19@leapchem.com | |||
 |
QQ chat | |||
| Chemical manufacturer since 2006 | ||||
| chemBlink standard supplier since 2015 | ||||
| Shanghai Qinba Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (21) 6517-0832 +86 17301729617 | |||
 |
shangfluoro@hotmail.com | |||
 |
Skype Chat | |||
 |
QQ chat | |||
| Chemical distributor since 2011 | ||||
| chemBlink standard supplier since 2017 | ||||
| Hangzhou Hairui Chemical Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 (571) 8669-1155 | |||
 |
sales@hairuichem.com | |||
| Chemical distributor since 2005 | ||||
| chemBlink standard supplier since 2017 | ||||
| Lavin Chemical (nantong) Co., Ltd. | China | Inquire | ||
|---|---|---|---|---|
 |
+86 13862887077 | |||
 |
lavin@lavinchem.com | |||
| Chemical distributor since 2012 | ||||
| chemBlink standard supplier since 2025 | ||||
| Classification | Organic raw materials >> Organic fluorine compound >> Fluorobenzoic acid series |
|---|---|
| Name | 1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one |
| Molecular Structure | 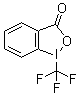 |
| Molecular Formula | C8H4F3IO2 |
| Molecular Weight | 316.02 |
| CAS Registry Number | 887144-94-7 |
| EC Number | 688-223-5 |
| SMILES | C1=CC=C2C(=C1)C(=O)OI2C(F)(F)F |
| Melting point | 150 - 158 ºC (Expl.) |
|---|---|
| Hazard Symbols |
 GHS07 Warning Details
GHS07 Warning Details | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Hazard Statements | H315-H319-H335 Details | ||||||||||||||||||||||||||||
| Precautionary Statements | P261-P264-P264+P265-P271-P280-P302+P352-P304+P340-P305+P351+P338-P319-P321-P332+P317-P337+P317-P362+P364-P403+P233-P405-P501 Details | ||||||||||||||||||||||||||||
| Hazard Classification | |||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||
| SDS | Available | ||||||||||||||||||||||||||||
|
1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one is a hypervalent iodine compound with the molecular formula C8H4F3IO2. Structurally, it features a five-membered 1,2-benziodoxole ring in which the iodine atom is in a +3 oxidation state, bonded to a carbonyl oxygen at the 3-position and a trifluoromethyl group at the 1-position. The fused benzene ring stabilizes the hypervalent iodine center, and the trifluoromethyl substituent imparts strong electron-withdrawing character. The compound typically appears as a crystalline solid, soluble in polar organic solvents such as acetonitrile, dichloromethane, and dimethylformamide, while being sensitive to moisture and light. This compound is primarily used as a trifluoromethylation reagent in organic synthesis. Its hypervalent iodine center enables the transfer of the –CF3 group to nucleophilic carbon centers, facilitating the introduction of trifluoromethyl groups into arenes, alkenes, and heterocycles under mild conditions. Trifluoromethylated products are highly valued in medicinal chemistry and agrochemicals because the –CF3 group can significantly enhance metabolic stability, lipophilicity, and bioavailability. 1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one is also employed in photoredox and transition-metal catalyzed reactions, where it serves as an electrophilic CF3 source. The reagent allows selective functionalization of complex molecules, making it a versatile tool for late-stage trifluoromethylation in drug discovery and materials chemistry. The compound is typically synthesized by oxidation of 2-iodobenzoic acid derivatives to the corresponding hypervalent iodine intermediate, followed by introduction of the trifluoromethyl group using suitable CF3 sources, such as trimethyl(trifluoromethyl)silane in the presence of fluoride activators. Handling requires standard precautions for hypervalent iodine compounds, including avoidance of friction, heat, and moisture, as they can be sensitive and potentially explosive under improper conditions. Overall, 1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one is a highly useful hypervalent iodine reagent that combines a stable benziodoxole framework with an electrophilic trifluoromethyl group. Its ability to transfer –CF3 efficiently under mild conditions makes it an important tool in modern synthetic organic chemistry, particularly for the preparation of trifluoromethylated arenes, heterocycles, and functional materials. References 2022. Direct Trifluoromethylation of C(sp3)�H Bonds. Science of Synthesis. URL: SD-238-00134 2022. Trifluoromethylation of Prefunctionalized Alkanes. Science of Synthesis. URL: SD-238-00133 2010. Reactivity of a Hypervalent Iodine Trifluoromethylating Reagent toward THF: Ring Opening and Formation of Trifluoromethyl Ethers. The Journal of Organic Chemistry, 75(3). DOI: 10.1021/jo9025429 |
| Market Analysis Reports |
| List of Reports Available for 1-(Trifluoromethyl)-1,2-benziodoxol-3(1H)-one |